推荐产品
等級
pharmaceutical primary standard
agency
USP
API 家族
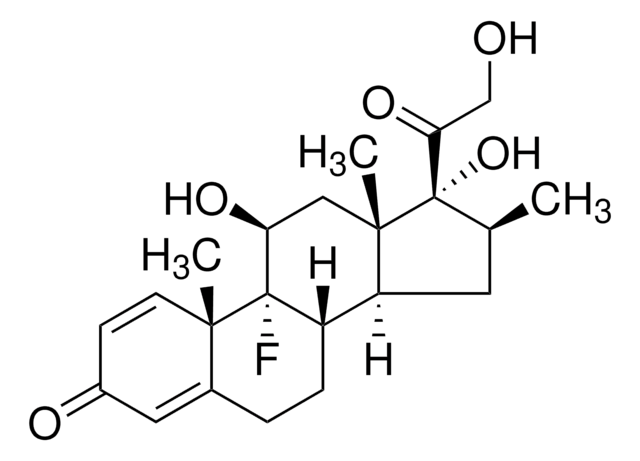
betamethasone
製造商/商標名
USP 1069095
應用
pharmaceutical
形式
mixture
儲存溫度
2-8°C
一般說明
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
USP issued SDS can be found here.
USP issued SDS can be found here.
應用
Betamethasone Valerate System Suitability Mixture USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia.
成分
A mixture containing betamethasone valerate and betamethasone valerate related compound H
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
訊號詞
Danger
危險聲明
危險分類
Repr. 1B - STOT RE 2
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 2
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门