推荐产品
等級
pharmaceutical primary standard
API 家族
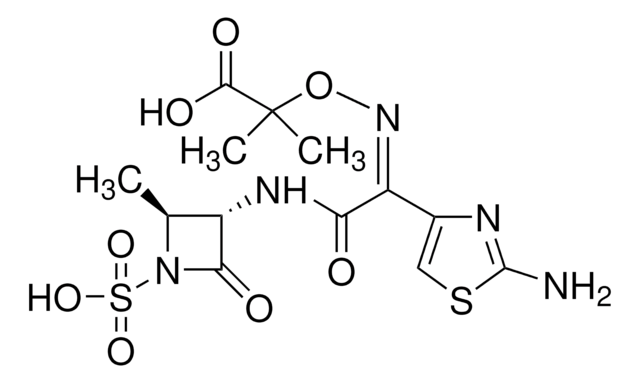
aztreonam
製造商/商標名
USP
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
−20°C
SMILES 字串
C[C@H]1[C@H](NC(=O)\C(=N/OC(C)(C)C(O)=O)c2csc(N)n2)C(=O)N1S(O)(=O)=O
InChI
1S/C13H17N5O8S2/c1-5-7(10(20)18(5)28(23,24)25)16-9(19)8(6-4-27-12(14)15-6)17-26-13(2,3)11(21)22/h4-5,7H,1-3H3,(H2,14,15)(H,16,19)(H,21,22)(H,23,24,25)/b17-8-/t5-,7-/m0/s1
InChI 密鑰
WZPBZJONDBGPKJ-VEHQQRBSSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Aztreonam USP reference standard is meant to use for specified quality tests and assays.
Also used to prepare standard and system suitability solutions for assay according to the given below monographs of United States Pharmacopeia (USP):
Also used to prepare standard and system suitability solutions for assay according to the given below monographs of United States Pharmacopeia (USP):
- Aztreonam
- Aztreonam for Injection
- Aztreonam Injection
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Aztreonam Injection
United States Pharmacopeia and National Formulary
United States Pharmacopeia, 39(3), 462-462 (2018)
Aztreonam for Injection
United States Pharmacopeia and National Formulary
United States Pharmacopeia, 39(3), 463-463 (2018)
Baroukh Maurice Assael
Expert review of anti-infective therapy, 9(11), 967-973 (2011-10-28)
An aerosol form of aztreonam lysinate has recently been developed as a treatment for cystic fibrosis patients suffering from chronic Pseudomonas aeruginosa lung colonization. Local administration means the drug can reach mucus concentrations in the order of hundreds of times
D H Johnson et al.
The Medical clinics of North America, 79(4), 733-743 (1995-07-01)
Aztreonam is a monocyclic beta-lactam antibiotic that is active exclusively against the aerobic gram-negative bacilli. It is not ototoxic or nephrotoxic and so is used as an alternative to aminoglycosides in a variety of clinical situations. In polymicrobial infections or
Kimberly A Pesaturo et al.
The Annals of pharmacotherapy, 46(7-8), 1076-1085 (2012-07-06)
To evaluate the pharmacology, clinical efficacy, and safety of aztreonam lysine for inhalation (AZLI) for cystic fibrosis (CF)-related signs and symptoms of pulmonary disease. Literature was searched in MEDLINE through PubMed and cross-referenced with EMBASE (1980-June 2012). The key search
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门