推荐产品
形狀
solid
品質等級
顏色
off-white
溶解度
DMF: soluble
儲存溫度
2-8°C
SMILES 字串
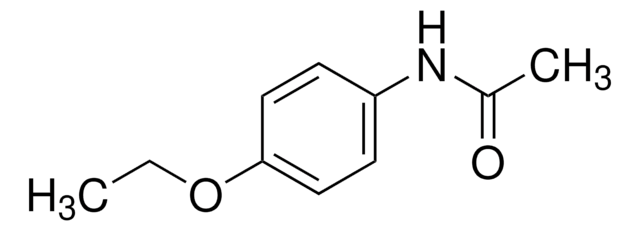
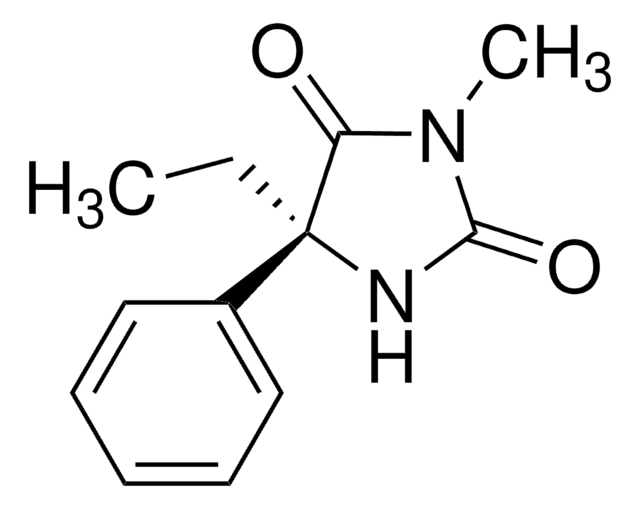
CC[C@@]1(NC(=O)NC1=O)c2ccccc2
InChI
1S/C11H12N2O2/c1-2-11(8-6-4-3-5-7-8)9(14)12-10(15)13-11/h3-7H,2H2,1H3,(H2,12,13,14,15)/t11-/m1/s1
InChI 密鑰
UDTWZFJEMMUFLC-LLVKDONJSA-N
生化/生理作用
CYP2B6 metabolite of (S)-(+)-mephenytoin; anticonvulsant; hypnotic.
包裝
Bottomless glass bottle. Contents are inside inserted fused cone.
準備報告
Nirvanol is soluble in DMF.
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
W Kalow
Xenobiotica; the fate of foreign compounds in biological systems, 16(5), 379-389 (1986-05-01)
The antiepileptic drug mephenytoin is a racemate. Mephenytoin hydroxylation is a stereospecific reaction and is confined to the S-enantiomer, which is normally eliminated within hours, allowing the R-enantiomer to accumulate since it can be eliminated only within days or weeks.
W H Theodore et al.
Neurology, 34(8), 1100-1102 (1984-08-01)
We investigated the conversion of mephenytoin to nirvanol in five patients with uncontrolled complex partial seizures. After a 50-mg single oral dose, mean peak mephenytoin level was 0.48 microgram/ml and nirvanol 0.37 microgram/ml. After 400 mg, peak mephenytoin level was
S H Akrawi et al.
European journal of drug metabolism and pharmacokinetics, 14(4), 269-278 (1989-10-01)
The stereoselective clearances of R- and S-mephenytoin were determined in rats receiving either an intravenous or hepatic portal vein infusion of racemic mephenytoin. The mean +/- SD intravenous clearances of R- and S-mephenytoin were 1630 +/- 250 ml/hr and 630
A Pezeshk et al.
Journal of inorganic biochemistry, 42(4), 267-272 (1991-06-01)
The preparation and spectral properties of copper(II) complexes of two hydantoins are reported. Complexes of the general formula Cu(hyd)2(py)2, where hyd = phenytoin or nirvanol; and py = pyridine were prepared and characterized by infrared and ESR. Spectral data show
B F Bourgeois et al.
Epilepsia, 27(4), 412-418 (1986-07-01)
Stereoselective metabolism has been demonstrated for mephenytoin (MHT), R-MHT being demethylated to the pharmacologically active metabolite 5-phenyl-5-ethylhydantoin (PEH; nirvanol), and S-MHT undergoing aromatic hydroxylation to 4-OH-MHT, with formation of an intermediate arene oxide metabolite. PEH is responsible for the therapeutic
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门