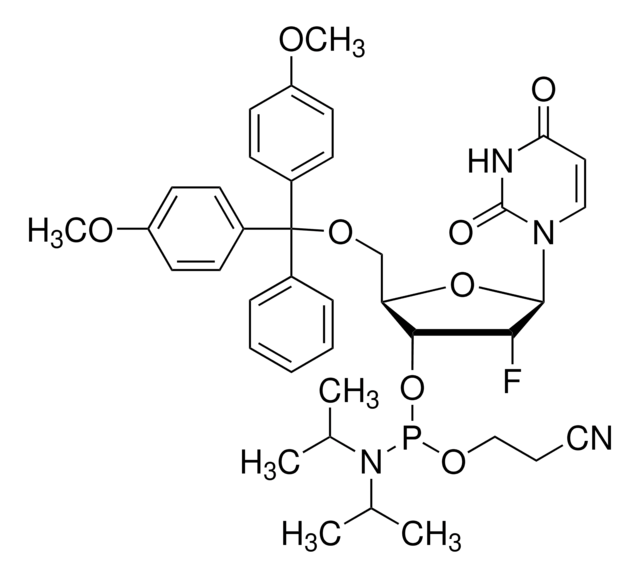
推荐产品
生物源
non-animal source (no BSE/TSE risk)
品質等級
產品線
Proligo Reagents
化驗
≥99% (31P-NMR)
≥99.0% (reversed phase HPLC)
形狀
powder
技術
oligo synthesis: suitable
雜質
≤0.1% single unspecified Impurity (reversed phase HPLC)
≤0.3% mU2 (reversed phase HPLC, Hydrolysate)
≤0.3% mU3 (reversed phase HPLC, DMT-rme)
≤0.3% water content (Karl Fischer)
≤0.5% P(III) Impurities 100-169ppm (31P-NMR)
≤1.0% mU1 (reversed phase HPLC, DMT-rUme-DMT)
≤3% residual Solvent content
顏色
white to off-white
&lambda ;
conforms (UV/VIS Identity)
適合性
conforms to structure for H-NMR
conforms to structure for LC-MS
相容性
configured for ÄKTA® and OligoPilot®
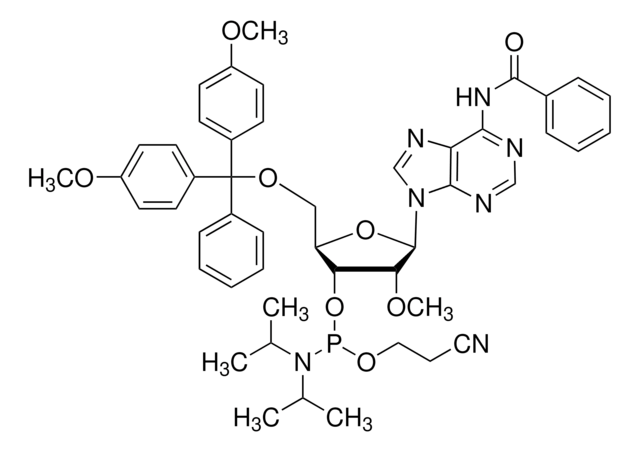
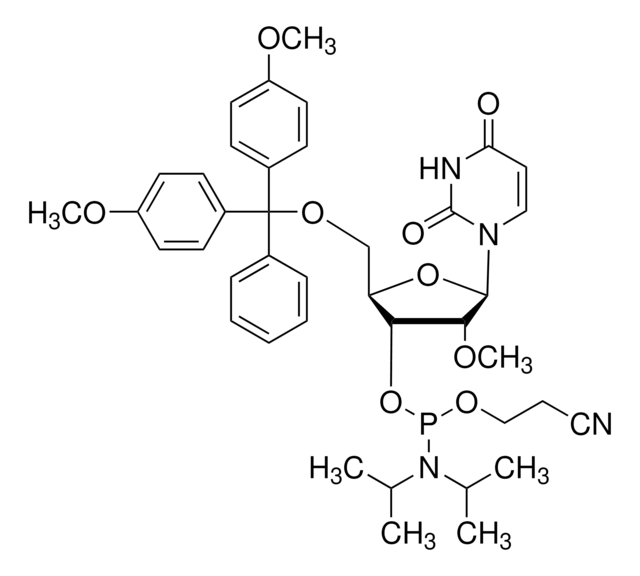
核苷譜
base: uridine
base protecting group: none
2' protecting group: methyl
5' protecting group: DMT
deprotection: fast/standard
儲存溫度
2-8°C
SMILES 字串
CO[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(c2ccccc2)(c3ccc(OC)cc3)c4ccc(OC)cc4)O[C@H]1N5C=CC(=O)NC5=O
InChI
1S/C40H49N4O9P/c1-27(2)44(28(3)4)54(51-25-11-23-41)53-36-34(52-38(37(36)49-7)43-24-22-35(45)42-39(43)46)26-50-40(29-12-9-8-10-13-29,30-14-18-32(47-5)19-15-30)31-16-20-33(48-6)21-17-31/h8-10,12-22,24,27-28,34,36-38H,11,25-26H2,1-7H3,(H,42,45,46)/t34-,36-,37-,38-,54?/m1/s1
InChI 密鑰
UVUOJOLPNDCIHL-XKZJCBTISA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
應用
特點和優勢
- High yield of crude oligonucleotides
- Compatible with DNA synthesis
- Can be employed together with DNA or RNA phosphoramidites in the same synthesis to produce mixmer oligonucleotides
- Recommended deprotection conditions are 8 hours at 55 °C using concentrated ammonia solution, or with AMA (concentrated ammonia/ 40% aqueous methylamine I/I, v/v) for 10 minutes at 65 °C
- Purification and other downstream processing of fully modified 2′OMethyl RNA oligonucleotides are simpler than in the case of RNA, as no special precautions are required to provide protection against nucleolytic degradation
- Synthesis of 2′O-Methyl RNA oligonucleotides is similar to standard DNA synthesis but requires an elongated coupling time (recommended is 6 minutes compared to 90 seconds for DNA monomers)
- 2′O-Methyl RNA phosphoramidites are also available with fast deprotection chemistry
其他說明
- Diagnostic probes
- Aptamer and ribozyme development
- Mixed 2′O-Methyl-RNA/DNA antisense molecules
法律資訊
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门