推荐产品
品質等級
化驗
≥98% (HPLC)
形狀
powder
顏色
white to tan
溶解度
DMSO, heptane and xylene: ≥17 mg/mL
起源
Novartis
儲存溫度
−20°C
SMILES 字串
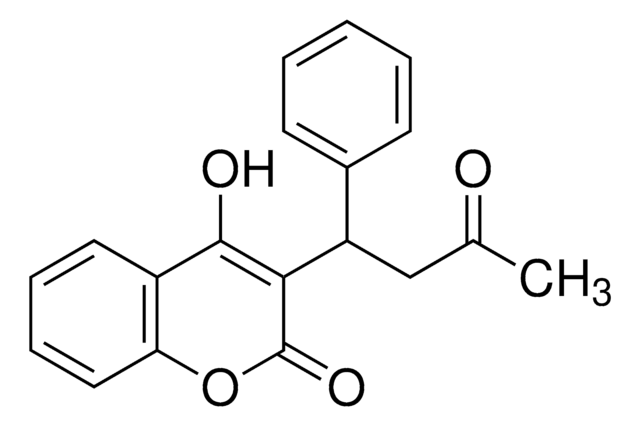
CC(=O)CC(c1ccc(cc1)[N+]([O-])=O)C2=C(O)c3ccccc3OC2=O
InChI
1S/C19H15NO6/c1-11(21)10-15(12-6-8-13(9-7-12)20(24)25)17-18(22)14-4-2-3-5-16(14)26-19(17)23/h2-9,15,22H,10H2,1H3
InChI 密鑰
VABCILAOYCMVPS-UHFFFAOYSA-N
基因資訊
human ... VKORC1(79001)
正在寻找类似产品? 访问 产品对比指南
應用
Acenocoumarol has been used as a standard for the determination of coumarins in cosmetics.
Acenocoumarol was used to study the role of P-glycoprotein in transport of oral vitamin K in Caco-2 cells and as an LC/MS standard.
生化/生理作用
Acenocoumarol is a warfarin analog, an anticoagulant that inhibits Vitamin K epoxide reductase. This results in depletion of the reduced form of vitamin K (vitamin KH2), limiting the gamma-carboxylation and subsequent activation of the vitamin K-dependent coagulation factors II, VII, IX, and X and anticoagulant proteins C and S, resulting in decreased prothrombin levels and the amount of thrombin generated.
Acenocoumarol is effective against thromboembolic disorders.
Warfarin analog; anticoagulant; inhibitor of Vit K epoxide reductase
特點和優勢
This compound was developed by Novartis. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Saurabh Singh Rathore et al.
PloS one, 7(5), e37844-e37844 (2012-05-26)
To develop a population specific pharmacogenetic acenocoumarol dosing algorithm for north Indian patients and show its efficiency in dosage prediction. Multiple and linear stepwise regression analyses were used to include age, sex, height, weight, body surface area, smoking status, VKORC1
Juan J Cerezo-Manchado et al.
Thrombosis and haemostasis, 109(1), 146-153 (2012-12-01)
Acenocoumarol is a commonly prescribed anticoagulant drug for the prophylaxis and treatment of venous and arterial thromboembolic disorders in several countries. In counterpart of warfarin, there is scarce information about pharmacogenetic algorithms for steady acenocoumarol dose estimation. The aim of
Determination of Coumarins in Cosmetics
Application Note, 1128 (2016)
Rianne M F van Schie et al.
Pharmacogenomics, 13(11), 1239-1245 (2012-08-28)
To evaluate the performance of the European Pharmacogenetics of Anticoagulant Therapy (EU-PACT) acenocoumarol dose algorithms in an independent data set. The EU-PACT trial investigates the added value of pretreatment genotyping for use of warfarin, phenprocoumon and acenocoumarol. External validation was
Alberto M Borobia et al.
PloS one, 7(7), e41360-e41360 (2012-08-23)
Appropriate dosing of coumarins is difficult to establish, due to significant inter-individual variability in the dose required to obtain stable anticoagulation. Several genetic and other clinical factors have been associated with the coumarins dose, and some pharmacogenetic-guided dosing algorithms for
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门