推荐产品
化驗
≥98% (HPLC)
形狀
powder
顏色
white to tan
溶解度
DMSO: ≥15 mg/mL
儲存溫度
room temp
SMILES 字串
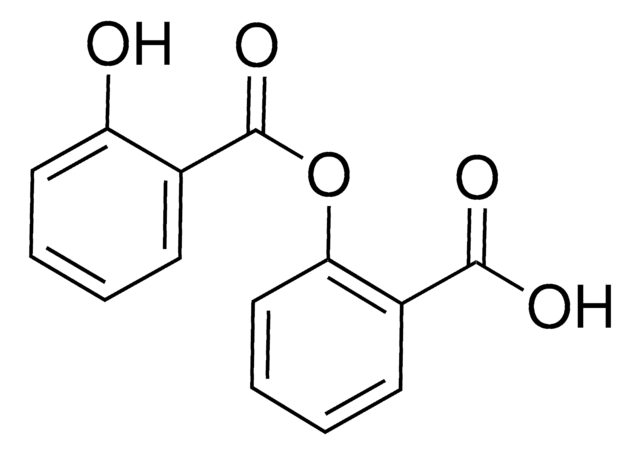
OC(=O)c1ccccc1OC(=O)c2ccccc2O
InChI
1S/C14H10O5/c15-11-7-3-1-5-9(11)14(18)19-12-8-4-2-6-10(12)13(16)17/h1-8,15H,(H,16,17)
InChI 密鑰
WVYADZUPLLSGPU-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
Salsalate was used to study the effect on palmitate-induced insulin resistance and hepatic steatosis in obese rats.
生化/生理作用
Salsalate is a nonsteroidal anti-inflammatory drug (NSAID), a nonacetylated salicylate with no more problems of gastrointestinal bleeding than placebo. It inhibits synthesis and release of prostaglandins through the inactivation of cyclooxygenase-1 (COX-1) and COX-2. Salsalate is currently being investigated as a treatment for Type 2 diabetes with possible use to prevent the disease in people at risk. It reduces blood glucose concentrations in patients with type 2 diabetes, as well as in insulin-resistant patients without diabetes.
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Martha M Rumore et al.
The Annals of pharmacotherapy, 44(7-8), 1207-1221 (2010-06-03)
To review the evidence base supporting the use of salicylates for glucose level control in patients with type 2 diabetes and provide a comprehensive review of available information describing the potential role of salicylates and, in particular, salsalate, for glucose
Zaichang Yang et al.
Phytomedicine : international journal of phytotherapy and phytopharmacology, 17(2), 139-141 (2009-09-15)
Therapeutic control of beta-lactamase-producing bacteria has been a major clinical problem. Development of drug combinations containing the beta-lactamase inhibitors has given clinicians a novel approach to controlling resistant organisms. In our search for beta-lactamase inhibitors from natural resources, we found
Allison B Goldfine et al.
Clinical and translational science, 1(1), 36-43 (2009-04-02)
Chronic subacute inflammation is implicated in the pathogenesis of insulin resistance and type 2 diabetes. Salicylates were shown years ago to lower glucose and more recently to inhibit NF-kappaB activity. Salsalate, a prodrug form of salicylate, has seen extensive clinical
Ruth C R Meex et al.
The Journal of clinical endocrinology and metabolism, 96(5), 1415-1423 (2011-02-04)
Nonsteroidal antiinflammatory drugs appear to improve insulin sensitivity and are currently tested in clinical trials. Salsalate, however, may blunt mitochondrial function, an unwarranted side effect for type 2 diabetics. We examined the effect of salsalate on ex vivo mitochondrial function
Weidong Chai et al.
Diabetes care, 34(7), 1634-1638 (2011-05-28)
Insulin recruits muscle microvasculature, thereby increasing endothelial exchange surface area. Free fatty acids (FFAs) cause insulin resistance by activating inhibitor of κB kinase β. Elevating plasma FFAs impairs insulin's microvascular and metabolic actions in vivo. Whether salsalate, an anti-inflammatory agent
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门