所有图片(1)
About This Item
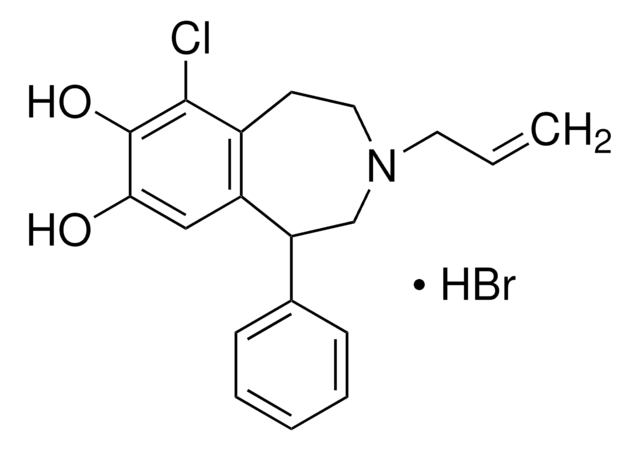
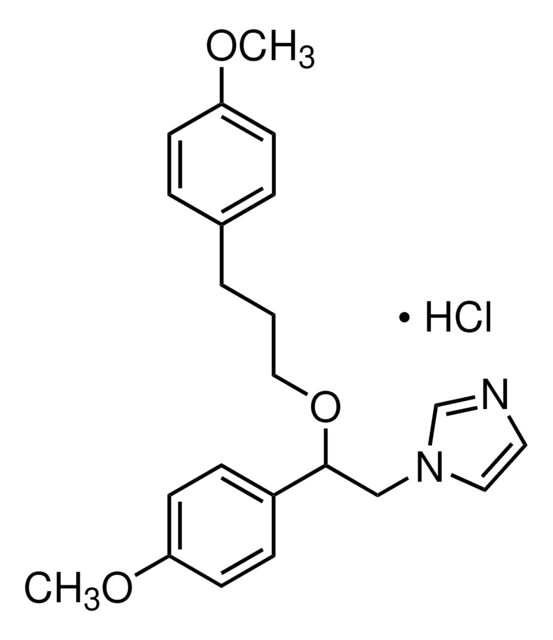
经验公式(希尔记法):
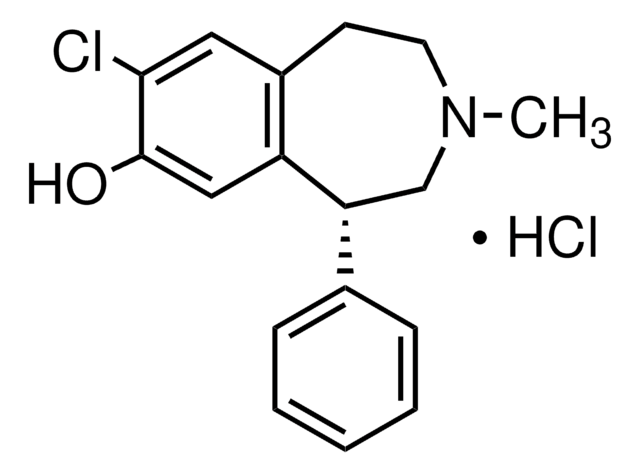
C16H17NO2 · HCl
CAS号:
分子量:
291.77
MDL號碼:
分類程式碼代碼:
12352200
PubChem物質ID:
NACRES:
NA.77
推荐产品
品質等級
化驗
≥98% (HPLC)
形狀
solid
光學活性
[α]22/D +16.1°, c = 1.2 in methanol(lit.)
儲存條件
desiccated
顏色
off-white to light tan
溶解度
0.1 M HCl: 1.2 mg/mL
ethanol: 3.4 mg/mL
H2O: 5 mg/mL
aqueous base: unstable
SMILES 字串
Cl.Oc1cc2CCNC[C@H](c3ccccc3)c2cc1O
InChI
1S/C16H17NO2.ClH/c18-15-8-12-6-7-17-10-14(13(12)9-16(15)19)11-4-2-1-3-5-11;/h1-5,8-9,14,17-19H,6-7,10H2;1H/t14-;/m1./s1
InChI 密鑰
YEWHJCLOUYPAOH-PFEQFJNWSA-N
正在寻找类似产品? 访问 产品对比指南
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Carole Hyacinthe et al.
Neurobiology of disease, 63, 20-24 (2013-11-12)
Both excessive daytime sleepiness (EDS) and rapid eye movement (REM) sleep deregulation are part of Parkinson's disease (PD) non-motor symptoms and may complicate dopamine replacement therapy. We report here that dopamine agonists act differentially on sleep architecture in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
Steven J Cooper et al.
European journal of pharmacology, 532(3), 253-257 (2006-02-16)
Free-feeding rats meet much of their daily energy requirements by consuming food in meals during the nocturnal phase of the night/day cycle. Meal pattern analysis methodology has been developed to record the patterns of meal taken over a 24-h period
C Kaiser et al.
Journal of medicinal chemistry, 25(6), 697-703 (1982-06-01)
Resolution of the unique dopamine receptor agonist 2,3,4,5-tetrahydro-7,8-dihydroxy-1-phenyl-1H-3-benzazepine (1) was achieved by a stereospecific multistep conversion of the readily separated enantiomers of its O,O,N-trimethylated precursor 2. The absolute stereochemistry of the antipodes of 2-MeI was determined by single-crystal X-ray diffractometric
J L Katz et al.
Psychopharmacology, 107(2-3), 217-220 (1992-01-01)
The present study was designed to assess the behavioral similarity of the effects of prototype dopamine receptor-subtype selective agonists and cocaine. Squirrel monkeys (N = 4) were trained with food reinforcement to press one of two levers after administration of
E Ongini et al.
Life sciences, 37(24), 2327-2333 (1985-12-16)
The dopamine D-1 receptor agonist SKF 38393 dose-dependently (2.5-10 mg/kg) induced desynchronization of the electroencephalographic (EEG) activity and behavioral arousal in both rabbits and rats. Unlike apomorphine, SKF 38393 elicited no signs of stereotyped behavior in rabbits and minimal effects
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门