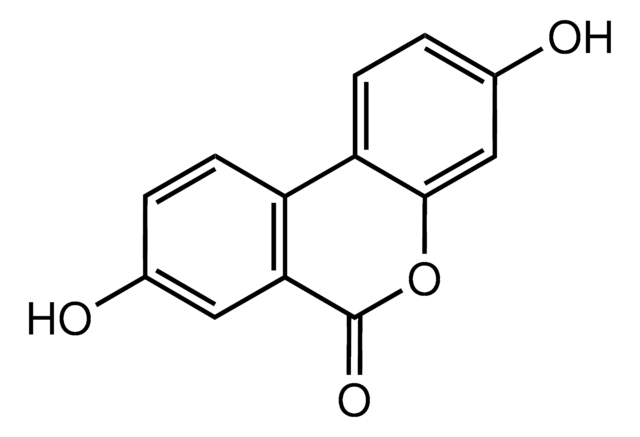
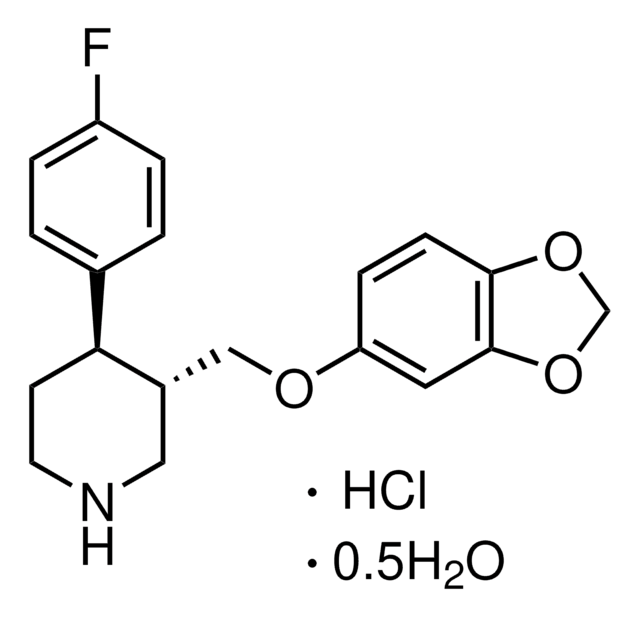
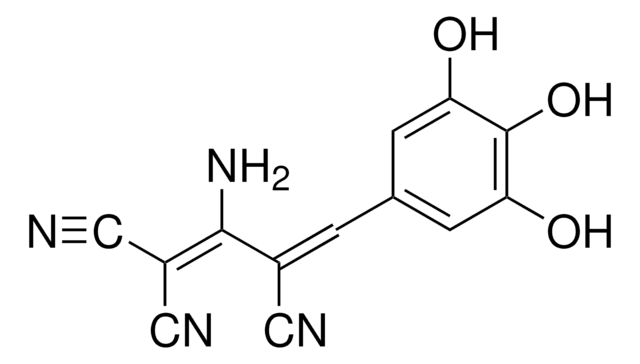
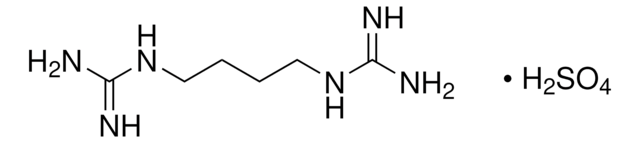
推荐产品
形狀
solid
顏色
white
溶解度
DMSO: >10 mg/mL
H2O: insoluble
起源
Servier
SMILES 字串
OC(=O)\C=C\C(O)=O.C1CN=C(NC(C2CC2)C3CC3)O1.C4CN=C(NC(C5CC5)C6CC6)O4
InChI
1S/2C10H16N2O.C4H4O4/c2*1-2-7(1)9(8-3-4-8)12-10-11-5-6-13-10;5-3(6)1-2-4(7)8/h2*7-9H,1-6H2,(H,11,12);1-2H,(H,5,6)(H,7,8)/b;;2-1+
InChI 密鑰
LZFATBMLSYHRTC-WXXKFALUSA-N
基因資訊
human ... NISCH(11188)
生化/生理作用
Selective I1 imidazoline receptor agonist.
特點和優勢
This compound is featured on the Imidazoline Binding Sites page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
This compound was developed by Servier. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
K Takada et al.
British journal of pharmacology, 120(8), 1575-1581 (1997-04-01)
1. To elucidate the possible involvement of pertussis toxin (PTX)-sensitive G proteins in the post receptor mechanism of alpha 2-adrenoceptors and imidazoline receptors, we examined the effect of pretreatment of the central nervous system with PTX on the antidysrhythmic effect
C K Chan et al.
The Journal of pharmacology and experimental therapeutics, 276(2), 411-420 (1996-02-01)
The present study in conscious rabbits with intracisternal (i.c.) catheters sought to determine the relative contribution of the I1 subtype of imidazoline receptors (IR) and alpha 2 adrenoceptors to the hypotensive effects of rilmenidine, clonidine and moxonidine with an I1-IR/alpha
M A Ozog et al.
Journal of neurochemistry, 71(4), 1429-1435 (1998-09-29)
Rilmenidine, a ligand for imidazoline and alpha2-adrenergic receptors, is neuroprotective following focal cerebral ischemia. We investigated the effects of rilmenidine on cytosolic free Ca2+ concentration ([Ca2+]i) in rat astrocytes. Rilmenidine caused concentration-dependent elevation of [Ca2+]i, consisting of a transient increase
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门