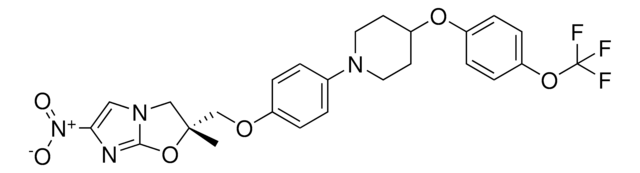
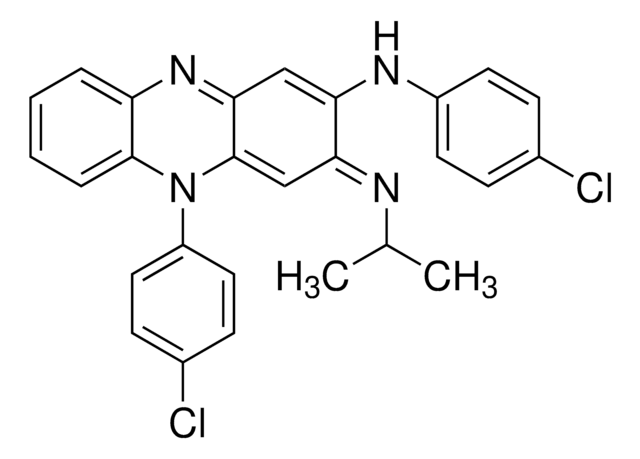
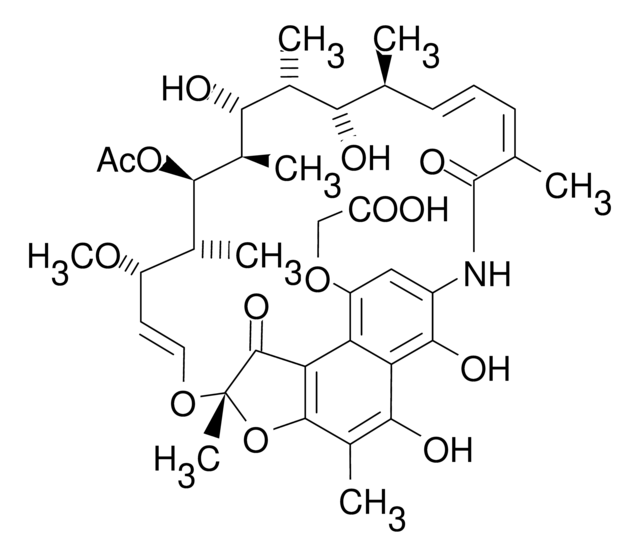
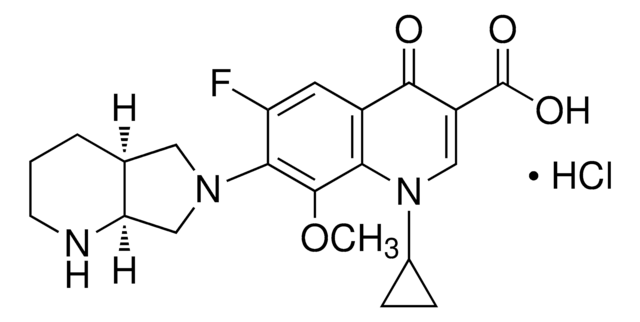
推荐产品
化驗
≥93.5%
品質等級
形狀
powder or crystals
抗生素活性譜
Gram-negative bacteria
Gram-positive bacteria
mycobacteria
作用方式
enzyme | inhibits
運輸包裝
wet ice
儲存溫度
2-8°C
InChI
1S/C47H64N4O12/c1-24-13-12-14-25(2)46(59)49-37-32(23-48-51-20-18-50(19-21-51)31-15-10-11-16-31)41(56)34-35(42(37)57)40(55)29(6)44-36(34)45(58)47(8,63-44)61-22-17-33(60-9)26(3)43(62-30(7)52)28(5)39(54)27(4)38(24)53/h12-14,17,22-24,26-28,31,33,38-39,43,53-57H,10-11,15-16,18-21H2,1-9H3,(H,49,59)/b13-12+,22-17+,25-14-,48-23?/t24-,26+,27+,28+,33?,38-,39+,43+,47-/m0/s1
InChI 密鑰
WDZCUPBHRAEYDL-OABFQHKQSA-N
一般說明
Chemical structure: macrolide
應用
Rifapentine is an antibiotic clinically used to treat tuberculosis. It is used in tuberculosis research.
生化/生理作用
Rifapentine is a semisynthetic derivative of rifampicin with antibacterial activity against Gram-positive and Gram-negative bacteria and against Mycobacterium tuberculosis. Rifapentine inhibits DNA-dependant RNA polymerase and prevents RNA transcription. It interacts with bacterial RNA polymerase but does not inhibit the mammalian enzyme.
Semisynthetic derivative of rifampicin with antibacterial activity against Gram-positive and Gram-negative bacterial and against Mycobacterium tuberculosis. Rifapentine inhibits DNA-dependant RNA polymerase and prevents RNA transcription.
其他說明
Keep container tightly closed in a dry and well-ventilated place.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Deepak Almeida et al.
PLoS neglected tropical diseases, 5(1), e933-e933 (2011-01-20)
treatment of Mycobacterium ulcerans disease, or Buruli ulcer (BU), has shifted from surgery to treatment with streptomycin(STR)+rifampin(RIF) since 2004 based on studies in a mouse model and clinical trials. We tested two entirely oral regimens for BU treatment, rifampin(RIF)+clarithromycin(CLR) and
K E Dooley et al.
Clinical pharmacology and therapeutics, 91(5), 881-888 (2012-04-05)
Rifapentine (RP T) is an antituberculosis drug that may shorten treatment duration when substituted for rifampin (RI F).The maximal tolerated daily dose of RP T and its potential for cytochrome 3A4 induction and autoinduction at clinically relevant doses are unknown.
John Gar Yan Chan et al.
Expert opinion on drug delivery, 11(3), 421-431 (2014-01-09)
Tuberculosis (TB) remains rampant throughout the world, in large part due to the lengthy treatment times of current therapeutic options. Rifapentine, a rifamycin antibiotic, is currently approved for intermittent dosing in the treatment of TB. Recent animal studies have shown
Ian M Rosenthal et al.
Antimicrobial agents and chemotherapy, 56(8), 4331-4340 (2012-06-06)
In previous experiments, replacing the 10-mg/kg of body weight daily dose of rifampin with 7.5 to 10 mg/kg of rifapentine in combinations containing isoniazid and pyrazinamide reduced the duration of treatment needed to cure tuberculosis in BALB/c mice by approximately
Ming Zhang et al.
American journal of respiratory and critical care medicine, 183(9), 1254-1261 (2011-02-19)
Daily rifapentine plus isoniazid-pyrazinamide in mice infected with Mycobacterium tuberculosis produces cure in 3 months. Whether cure corresponds to latent infection contained by host immunity or true tissue sterilization is unknown. To determine the length of treatment with rifapentine-isoniazid-pyrazinamide or
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门