推荐产品
产品名称
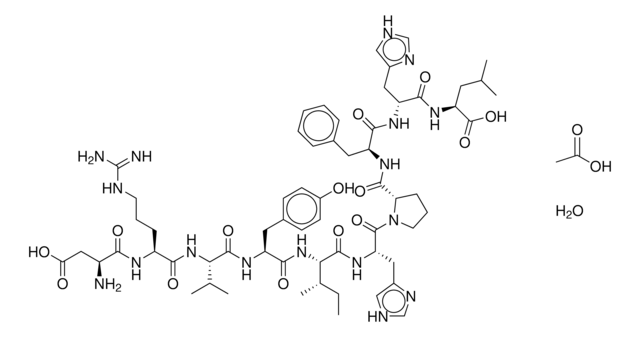
Neurotensin Fragment 8-13 acetate salt, ≥97% (HPLC)
化驗
≥97% (HPLC)
形狀
powder
技術
ligand binding assay: suitable
顏色
white
應用
cell analysis
儲存溫度
−20°C
SMILES 字串
CCC(C)C(NC(=O)C(Cc1ccc(O)cc1)NC(=O)C2CCCN2C(=O)C(CCCNC(N)=N)NC(=O)C(N)CCCNC(N)=N)C(=O)NC(CC(C)C)C(O)=O
InChI
1S/C38H64N12O8/c1-5-22(4)30(34(55)48-28(36(57)58)19-21(2)3)49-32(53)27(20-23-12-14-24(51)15-13-23)47-33(54)29-11-8-18-50(29)35(56)26(10-7-17-45-38(42)43)46-31(52)25(39)9-6-16-44-37(40)41/h12-15,21-22,25-30,51H,5-11,16-20,39H2,1-4H3,(H,46,52)(H,47,54)(H,48,55)(H,49,53)(H,57,58)(H4,40,41,44)(H4,42,43,45)
InChI 密鑰
DQDBCHHEIKQPJD-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
相关类别
Amino Acid Sequence
Arg-Arg-Pro-Tyr-Ile-Leu
生化/生理作用
Neurotensin Fragment 8-13 acetate salt is the smallest active fragment of neurotensin.
聯結
Smallest active fragment of neurotensin
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
M Kyle Hadden et al.
Neuropharmacology, 49(8), 1149-1159 (2005-08-13)
Neurotensin (NT) and its active fragment NT(8-13) elicit behavioral responses typical of clinically used antipsychotic drugs when administered directly to the brain. However, limited peptide stability and oral bioavailability have prevented these compounds from being developed as relevant pharmaceuticals. Recently
Kevin S Orwig et al.
Journal of medicinal chemistry, 52(7), 1803-1813 (2009-03-18)
Neurotensin(8-13) and two related analogues were used as model systems to directly compare various N-terminal peptide modifications representing both commonly used and novel capping groups. Each N-terminal modification prevented aminopeptidase cleavage but surprisingly differed in its ability to inhibit cleavage
Takaaki Yanai et al.
Bioscience, biotechnology, and biochemistry, 67(2), 380-382 (2003-05-06)
Two peptides that inhibit prolyl endopeptidase were isolated from a red wine made from Cabernet Sauvignon grapes. Their amino acid sequences and IC50 values were Val-Glu-Ile-Pro-Glu (17.0 microM) and Tyr-Pro-Ile-Pro-Phe (87.8 microM). The peptides also suppressed the degradation levels of
Veronique Maes et al.
Journal of medicinal chemistry, 49(5), 1833-1836 (2006-03-03)
Two new 99mTc-labeled neurotensin(8-13) analogues containing the retro-N(alpha)-carboxymethyl-histidine ((N(alpha)His)Ac) chelator were synthesized as potential radiopharmaceuticals for visualization of pancreatic carcinoma. To improve the pharmacokinetic properties, (N(alpha)His)Ac-Arg-NMeArg-Pro-Tyr-Tle-Leu (NT-XII), which is metabolically stabilized at two positions, was further modified. Shikimic acid (3,4,5-trihydroxy-1-cyclohexene-1-carboxylic
Rodrigo Teodoro et al.
Nuclear medicine and biology, 38(1), 113-120 (2011-01-12)
Several strategies on the development of radiopharmaceuticals have been employed. Bifunctional chelators seem to be a promising approach since high radiochemical yields as well as good in vitro and in vivo stability have been achieved. To date, neurotensin analogs have
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门