所有图片(2)
About This Item
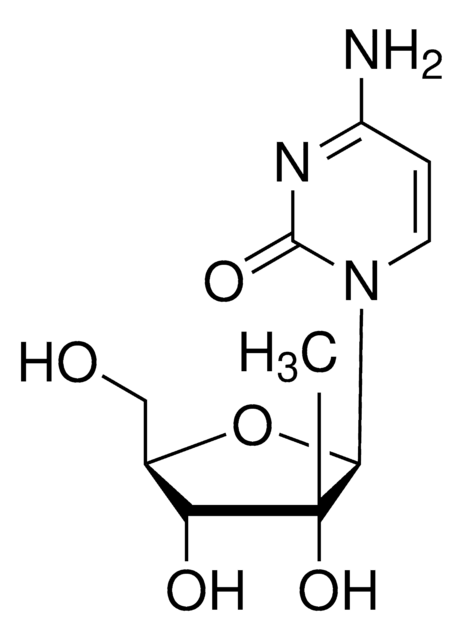
经验公式(希尔记法):
C9H13N3O6
CAS号:
分子量:
259.22
MDL號碼:
分類程式碼代碼:
12352200
PubChem物質ID:
NACRES:
NA.77
推荐产品
品質等級
化驗
≥98% (TLC)
儲存溫度
2-8°C
SMILES 字串
NC(=O)c1ncn([C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O)c1O
InChI
1S/C9H13N3O6/c10-7(16)4-8(17)12(2-11-4)9-6(15)5(14)3(1-13)18-9/h2-3,5-6,9,13-15,17H,1H2,(H2,10,16)/t3-,5-,6-,9-/m1/s1
InChI 密鑰
HZQDCMWJEBCWBR-UUOKFMHZSA-N
應用
Mizoribine has been used in topical treatment to evaluate its effect on ocular surface damage of dry eye in B6 mice subjected to desiccating stress (DS). It has also been used as an inosine monophosphate dehydrogenase (IMPDH) inhibitor to test its in vivo efficacy.
生化/生理作用
Mizoribine is an imidazole nucleoside possessing strong immunosuppressive properties. It selectively blocks T-cell proliferation response to mitogenic and allo-antigenic stimulation. Mizoribine blocks the movement of T cells from G to S phase. In addition, it significantly decreases the number of B cells at the S, G, and M phases. Mizoribine inhibits de novo synthesis of nucleotides by inhibition of inosine monophosphate dehydrogenase. The resulting nucleotide depletion inhibits DNA synthesis.(3)
訊號詞
Danger
危險分類
Eye Irrit. 2 - Repr. 1B - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
S Hirohata et al.
Journal of immunology (Baltimore, Md. : 1950), 155(11), 5175-5183 (1995-12-01)
Mizoribine has been used to prevent rejection of organ allografts in humans and in animal models. Recent clinical trials have demonstrated its efficacy in rheumatoid arthritis and lupus nephritis, in which abnormalities of B cell functions are also involved. We
C V Catapano et al.
Molecular pharmacology, 47(5), 948-955 (1995-05-01)
Inhibitors of IMP dehydrogenase (EC 1.2.1.14), including mizoribine (Bredinin) and mycophenolic acid, have significant antitumor and immunosuppressive activities. Studies were aimed at determining the mechanism by which intracellular GTP depletion induced by these agents results in inhibition of DNA synthesis.
Topical Application of Mizoribine Suppresses CD4+ T-cell-Mediated Pathogenesis in Murine Dry Eye
Zhang X, et al.
Investigative Ophthalmology & Visual Science, 58(14), 6056-6064 (2017)
Michitoshi Yamashita et al.
Cell transplantation, 21(2-3), 535-545 (2012-07-17)
Mizoribine (MZ) inhibits the differentiation and proliferation of helper T and B cells after antigen recognition by suppressing the purine biosynthesis pathway and nucleic acid synthesis. MZ has been used in kidney transplantation, but distinct data are unavailable for islet
Hideo Ohtsubo et al.
Modern rheumatology, 22(6), 837-843 (2012-03-07)
The efficacy of mizoribine (MZR) in treatment of rheumatoid arthritis (RA) was retrospectively investigated in terms of drug survival, improvement in Disease Activity Score-28 (DAS28)-C-reactive protein (CRP), and blood MZR concentration obtained 3 h after dosing (MZR-C3). To compare the efficacy
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门