所有图片(1)
About This Item
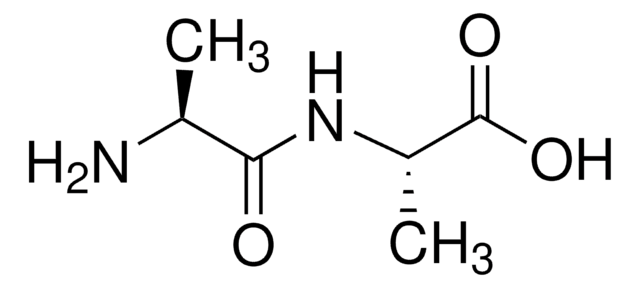
线性分子式:
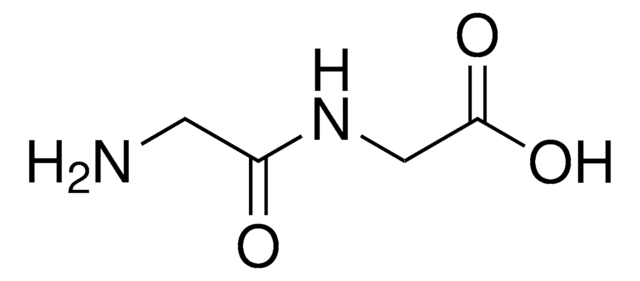
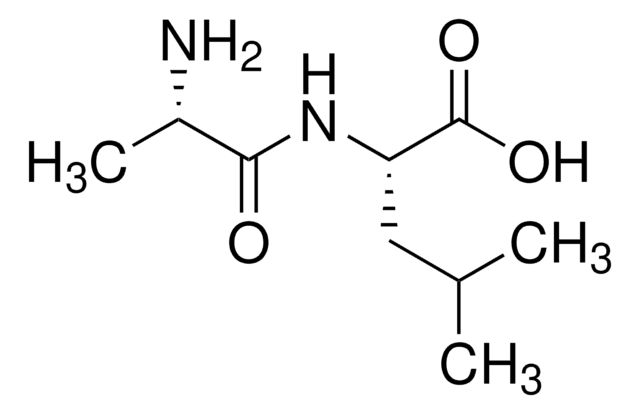
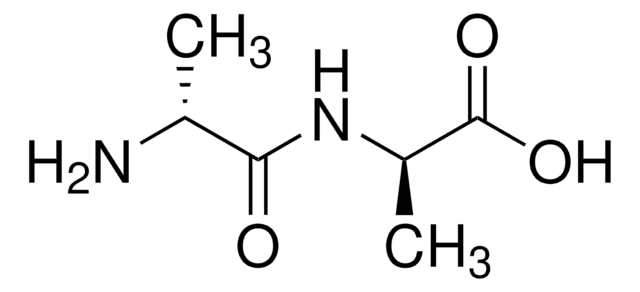
(CH3)2CHCH2CH(NH2)CONHCH(CH3)COOH · xH2O
CAS号:
分子量:
202.25 (anhydrous basis)
Beilstein:
1726165
MDL號碼:
分類程式碼代碼:
12352209
PubChem物質ID:
NACRES:
NA.26
推荐产品
品質等級
化驗
≥98% (TLC)
形狀
powder
技術
ligand binding assay: suitable
顏色
white
儲存溫度
2-8°C
SMILES 字串
O.CC(C)C[C@H](N)C(=O)N[C@@H](C)C(O)=O
InChI
1S/C9H18N2O3.H2O/c1-5(2)4-7(10)8(12)11-6(3)9(13)14;/h5-7H,4,10H2,1-3H3,(H,11,12)(H,13,14);1H2/t6-,7-;/m0./s1
InChI 密鑰
FTDFAGANYJVHDA-LEUCUCNGSA-N
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
亮氨酰丙氨酸(Leu-Ala)被用于制备非电解三有机锡(IV)衍生物(通式 R3Sn(HL)),以研究金属蛋白相互作用的模型。
應用
亮氨酰丙氨酸 (Leu-Ala) 被用于制备非电解三有机锡 (IV) 衍生物(通式 R3Sn (HL)),以研究金属蛋白相互作用的模型。
以 Leu-Ala 水合物为底物,在三-盐酸(HCl)缓冲液中分析了幼虫中亮氨酸-丙氨酸肽酶(LAP)的活性。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
New triorganotin (IV) derivatives of dipeptides as models for metal-protein interactions: synthesis, structural characterization and biological studies.
Nath M, Pokharia S, Eng G, Song X, Kumar A.
Spectrochimica Acta Part A: Molecular Spectroscopy, 63, 66-75 (2006)
David S Milner et al.
Proceedings of the National Academy of Sciences of the United States of America, 116(12), 5613-5622 (2019-03-08)
Many microbes acquire metabolites in a "feeding" process where complex polymers are broken down in the environment to their subunits. The subsequent uptake of soluble metabolites by a cell, sometimes called osmotrophy, is facilitated by transporter proteins. As such, the
Arun K Ghosh et al.
Journal of medicinal chemistry, 50(10), 2399-2407 (2007-04-17)
Structure-based design and synthesis of a number of potent and selective memapsin 2 inhibitors are described. These inhibitors were designed based upon the X-ray structure of memapsin 2-bound inhibitor 3 that incorporates methylsulfonyl alanine as the P2-ligand and a substituted
L P Biały et al.
Folia histochemica et cytobiologica, 40(2), 135-136 (2002-06-12)
We have used the dipeptide Leu-Ala in an attempt to prevent the formation of ubiquitin-protein conjugates in U937 cells by inhibition of cellular E3 enzymes (ubiquitin ligases). Proteasome inhibitors induce the formation of perinuclear aggregates of ubiquitinated proteins and proteasomes
Marie Terpager et al.
Journal of receptor and signal transduction research, 29(5), 235-245 (2009-09-15)
7TM receptors are easily fused to proteins such as G proteins and arrestin but because of the fact that their terminals are found on each side of the membrane they cannot be joined directly in covalent dimers. Here, we use
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门