推荐产品
生物源
synthetic
品質等級
化驗
≥97% (HPLC)
形狀
powder
雜質
glucose, essentially free
顏色
white
溶解度
water: slightly soluble 50 g/L
儲存溫度
−20°C
SMILES 字串
[K+].[K+].[H]O[H].OC[C@H]1O[C@H](OP([O-])([O-])=O)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C6H13O9P.2K.H2O/c7-1-2-3(8)4(9)5(10)6(14-2)15-16(11,12)13;;;/h2-10H,1H2,(H2,11,12,13);;;1H2/q;2*+1;/p-2/t2-,3-,4+,5-,6-;;;/m1.../s1
InChI 密鑰
VOQGDSVKCMGEFO-FBNUBEQJSA-L
正在寻找类似产品? 访问 产品对比指南
一般說明
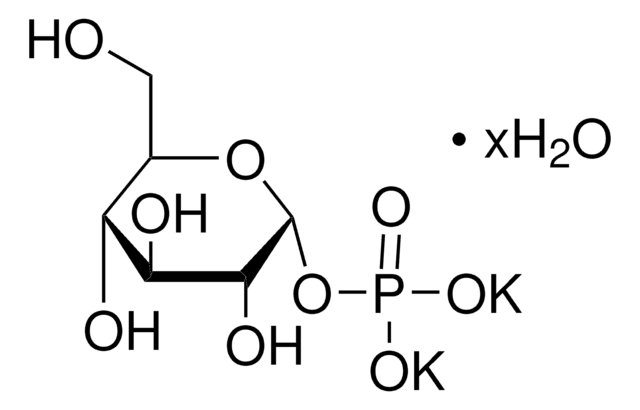
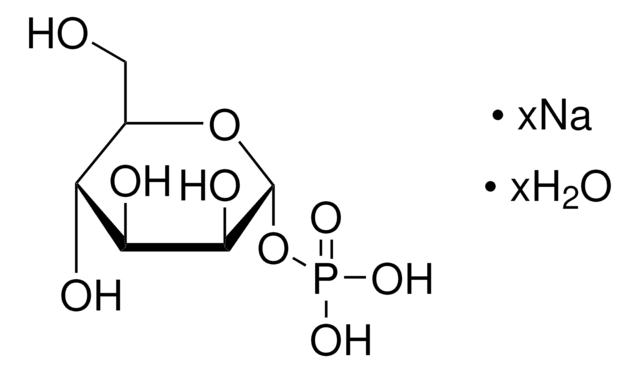
α-D-Glucose 1-phosphate is the α-anomeric form of glucose which contains a phosphate group on the primary carbon. It can be converted into the deoxysugar CDP-glucose by the enzyme α-D-Glucose-1-phosphate cytidylyltransferase.
聯結
Formerly listed as Grade I.
準備報告
Prepared by a modification of the procedure of McCready, R.M., et al., J. Am. Chem. Soc., 66, 560 (1944).
其他說明
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Bijay Singh et al.
Protein engineering, design & selection : PEDS, 25(4), 179-187 (2012-02-16)
Two similar genes, dnmL and rmbA in Streptomyces peucetius, which encode for glucose-1-phosphate (G-1-P) thymidylyltransferases were expressed in Escherichia coli under similar conditions. While RmbA was expressed in soluble form, DnmL was found as insoluble aggregates in inclusion bodies. The
Kyra-Melinda Alexacou et al.
Bioorganic & medicinal chemistry, 18(22), 7911-7922 (2010-10-16)
Glycogen phosphorylase (GP) is a promising target for the treatment of type 2 diabetes. In the process of structure based drug design for GP, a group of 15 aromatic aldehyde 4-(β-d-glucopyranosyl)thiosemicarbazones have been synthesized and evaluated as inhibitors of rabbit
Stanley A Blumenthal
Perspectives in biology and medicine, 55(2), 236-249 (2012-05-31)
In 1945, Earl Sutherland (1915-1974) [corrected] and associates began studies of the mechanism of hormone-induced glycogen breakdown in the liver. In 1956, their efforts culminated in the identification of cyclic AMP, an ancient molecule generated in many cell types in
Jef Van der Borght et al.
Biotechnology journal, 5(9), 986-993 (2010-08-28)
β-D-Glucose-1-phosphate (βGlc1P) is an efficient glucosyl donor for both enzymatic and chemical glycosylation reactions but is currently very costly and not available in large amounts. This article provides an efficient production method of βGlc1P from trehalose and phosphate using the
Cátia Nunes et al.
Plant physiology and biochemistry : PPB, 63, 89-98 (2012-12-22)
SnRK1 of the SNF1/AMPK group of protein kinases is an important regulatory protein kinase in plants. SnRK1 was recently shown as a target of the sugar signal, trehalose 6-phosphate (T6P). Glucose 6-phosphate (G6P) can also inhibit SnRK1 and given the
实验方案
This procedure may be used for all Phosphoglucomutase products except for Phosphoglucomutase, Catalog Number P4109.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门