推荐产品
生物源
bacterial (Proteus spp.)
品質等級
形狀
buffered aqueous solution
比活性
≥4,000 units/mL
分子量
~300 kDa
儲存溫度
2-8°C
應用
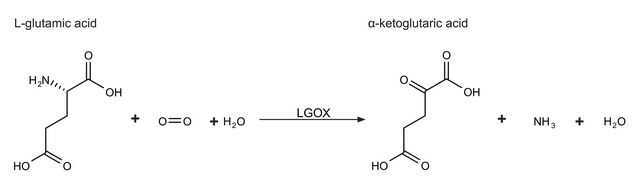
This enzyme is useful for enzymatic determination of NH3, α-ketoglutaric acid and L-glutamic acid, and for assay of leucine aminopeptidase and urease. This enzyme is also used for enzymatic determination of urea when coupled with urease (URH-201) in clinical analysis. In vitro, various activity assays of this enzyme examine the conversion of α-ketoglutarate to L-glutamate, in the presence of excess ammonium ions (NH4+) and NADPH.
生化/生理作用
L-glutamic dehydrogenase catalyzes the conversion of glutamate to α-ketoglutarate.
物理性質
Isoelectric point : 4.6
Michaelis constants : 1.1 X 10-3M (NH3), 3.4 X 10-4M (α-Ketoglutarate)
1.2 X 10-3M (L-Glutamate), 1.4 X 10-5M (NADPH), 1.5 X 10-5M (NADP+)
Structure : 6 subunits (M.W.50,000) per mol of enzyme
Inhibitors : Hg++, Cd++, p-chloromercuribenzoate, pyridine, 4-4′-dithiopyridine,
2,2′-dithiopyridine
Optimum pH : 8.5 (α-KG→L-Glu) 9.8 (L-Glu→α-KG)
Optimum temperature : 45oC(α-KG−L-Glu) 45-55oC (L-Glu→α-KG)
pH stability : pH 6.0 - 8.5 (25oC, 20hr)
Thermal stability : below 50oC (pH 7.4, 10min)
Michaelis constants : 1.1 X 10-3M (NH3), 3.4 X 10-4M (α-Ketoglutarate)
1.2 X 10-3M (L-Glutamate), 1.4 X 10-5M (NADPH), 1.5 X 10-5M (NADP+)
Structure : 6 subunits (M.W.50,000) per mol of enzyme
Inhibitors : Hg++, Cd++, p-chloromercuribenzoate, pyridine, 4-4′-dithiopyridine,
2,2′-dithiopyridine
Optimum pH : 8.5 (α-KG→L-Glu) 9.8 (L-Glu→α-KG)
Optimum temperature : 45oC(α-KG−L-Glu) 45-55oC (L-Glu→α-KG)
pH stability : pH 6.0 - 8.5 (25oC, 20hr)
Thermal stability : below 50oC (pH 7.4, 10min)
單位定義
One unit will reduce 1.0 μmole of α-ketoglutarate to L-glutamate per min at pH 8.3 at 30 °C in the presence of ammonium ions and NADPH.
外觀
Solution in 50 mM Tris HCl, pH 7.8, 5 mM Na2EDTA containing 0.05% sodium azide
其他說明
Note: Do not confuse with non-specific L-GLDH, EC 1.4.1.3.
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
J Bailey et al.
The Journal of biological chemistry, 257(10), 5579-5583 (1982-05-25)
The activity of bovine liver glutamate dehydrogenase is affected in several ways depending on substrate concentrations and pH. At ph 6.5 and below, both oxidative deamination and reductive amination reactions are inhibited by ADP. At pH 7.0 and above both
D P Hornby et al.
The Biochemical journal, 223(1), 161-168 (1984-10-01)
In steady-state kinetic studies of ox liver glutamate dehydrogenase in 0.11 M-potassium phosphate buffer, pH7, at 25 degrees C, the concentration of ADP was varied from 0.5 to 1000 microM. Inhibition was observed except when the concentrations of both glutamate
Daria V Borsakova et al.
Scientific reports, 12(1), 5437-5437 (2022-04-02)
Excessive ammonium blood concentration causes many serious neurological complications. The medications currently used are not very effective. To remove ammonium from the blood, erythrocyte-bioreactors containing enzymes that processing ammonium have been proposed. The most promising bioreactor contained co-encapsulated glutamate dehydrogenase
Tomomi Abiko et al.
Planta, 232(2), 299-311 (2010-05-06)
In plants, glutamine synthetase (GS) is the enzyme that is mainly responsible for the assimilation of ammonium. Conversely, in microorganisms such as bacteria and Ascomycota, NADP(H)-dependent glutamate dehydrogenase (GDH) and GS both have important roles in ammonium assimilation. Here, we
Daniel Juan Herrera et al.
Annals of clinical biochemistry, 47(Pt 1), 81-83 (2009-11-27)
Enzymatic assays using glutamate dehydrogenase (GLDH) to monitor the transformation of NAD(P)H to NAD(P)(+) by a spectrophotometric technique are the most common methods to measure plasma ammonia (PA) in routine laboratories worldwide. However, these assays can potentially be subject to
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门