推荐产品
产品名称
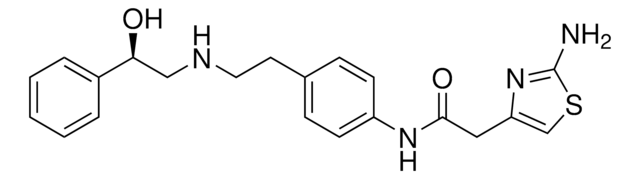
吡格列酮 盐酸盐, ≥98% (HPLC)
化驗
≥98% (HPLC)
形狀
powder
顏色
white to off-white
溶解度
DMSO: ≥10 mg/mL
起源
Takeda
儲存溫度
room temp
SMILES 字串
Cl.CCc1ccc(CCOc2ccc(CC3SC(=O)NC3=O)cc2)nc1
InChI
1S/C19H20N2O3S.ClH/c1-2-13-3-6-15(20-12-13)9-10-24-16-7-4-14(5-8-16)11-17-18(22)21-19(23)25-17;/h3-8,12,17H,2,9-11H2,1H3,(H,21,22,23);1H
InChI 密鑰
GHUUBYQTCDQWRA-UHFFFAOYSA-N
基因資訊
human ... PPARG(5468)
正在寻找类似产品? 访问 产品对比指南
一般說明
Pioglitazone hydrochloride consists of poly-morphs, form I and form II. It is an oral antidiabetic agent, that is a member of the thiazolidinedione group.
應用
Pioglitazone hydrochloride has been used:
- to administer to mice model and treated the hepatoma cell line to study its effect on regulating insulin-degrading enzyme (IDE) in diet-induced obese (DIO) C57BL/6 mice
- in drug preparation to analyze its effects on shortening and calcium transport in ventricular myocytes from the Goto-Kakizaki (GK) type 2 diabetic rat
- to treat HepG2 cells with peroxisome proliferator-activated receptor γ (PPARγ) agonists to examine its effect on TOMM40-, APOE- and APOC1-mRNA levels
生化/生理作用
盐酸吡格列酮是一种PPARγ激动剂和噻唑烷二酮(TZD)抗糖尿病药物。
盐酸吡格列酮是一种PPARγ激动剂和噻唑烷二酮(TZD)抗糖尿病药物。吡格列酮是一种核受体过氧化物酶体增殖物激活受体γ(PPAR-γ)的选择性激动剂,并在较小程度上是PPAR-α。
Pioglitazone hydrochloride is usually used to treat type-II diabetes. It has the ability to block hepatic gluconeogenesis.
特點和優勢
This compound is a featured product for ADME Tox research. Click here to discover more featured ADME Tox products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
This compound is featured on the AMPKs and Nuclear Receptors (PPARs) pages of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
This compound was developed by Takeda. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Carc. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Romina Lomonaco et al.
Drugs, 73(1), 1-14 (2013-01-19)
Nonalcoholic fatty liver disease (NAFLD) is considered the most common liver disorder in the Western world. It is commonly associated with insulin resistance, obesity, dyslipidaemia, type 2 diabetes mellitus (T2DM) and cardiovascular disease. Nonalcoholic steatohepatitis (NASH) is characterized by steatosis
Andrew Grey et al.
European journal of endocrinology, 170(2), 255-262 (2013-11-13)
Preclinical studies, observational studies, and clinical trials suggest that thiazolidinediones (TZDs) reduce bone mineral density (BMD) and increase fracture risk. Most of the evidence on the skeletal effects of TZDs is from studies of rosiglitazone. We set out to investigate
Peter Ochodnicky et al.
European journal of pharmacology, 730, 51-60 (2014-03-04)
Peroxisome proliferator-activated receptor γ (PPARγ) agonists have been shown to ameliorate diabetic nephropathy, but much less are known about their effects in non-diabetic nephropathies. In the present study, metabolic parameters, blood pressure, aortic endothelial function along with molecular and structural
J R Colca et al.
Clinical pharmacology and therapeutics, 93(4), 352-359 (2013-03-07)
It may be possible to achieve insulin sensitivity through the recently identified mitochondrial target of thiazolidinediones (mTOT), thereby avoiding peroxisome proliferator-activated receptor-γ (PPAR-γ)-dependent side effects. In this phase IIb clinical trial, 258 patients with type 2 diabetes completed a 12-week
Julien Lamontagne et al.
Diabetes, 62(6), 2122-2129 (2013-02-05)
Our objective was to determine if the insulin-sensitizing drug pioglitazone acutely reduces insulin secretion and causes metabolic deceleration in vivo independently of change in insulin sensitivity. We assessed glucose homeostasis by hyperinsulinemic-euglycemic and hyperglycemic clamp studies and energy expenditure by
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门