所有图片(1)
About This Item
经验公式(希尔记法):
C20H24O2
CAS号:
分子量:
296.40
Beilstein:
1798411
MDL號碼:
分類程式碼代碼:
12352106
PubChem物質ID:
NACRES:
NA.77
推荐产品
生物源
synthetic (organic)
化驗
≥97%
形狀
powder
mp
78-80 °C
溶解度
ethanol: 50 mg/mL, clear, colorless to yellow
儲存溫度
−20°C
SMILES 字串
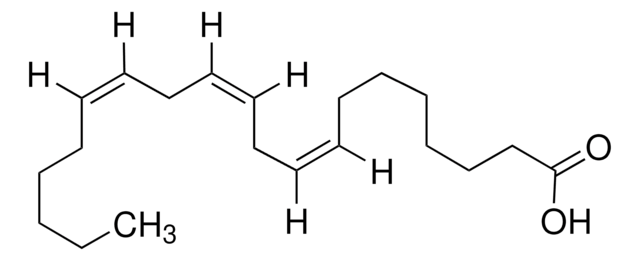
CCCCCC#CCC#CCC#CCC#CCCCC(O)=O
InChI
1S/C20H24O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20(21)22/h2-5,8,11,14,17-19H2,1H3,(H,21,22)
InChI 密鑰
MGLDCXPLYOWQRP-UHFFFAOYSA-N
基因資訊
human ... ALOX15(246)
正在寻找类似产品? 访问 产品对比指南
生化/生理作用
Eicosatetraynoic acid (ETYA) is a non-metabolizable analog of ω-6 arachidonic acid. ETYA is a strong activator of the human peroxisome proliferator-activated receptor α (PPARα). It acts as an inhibitor of lipoxygenases (LOX) and cyclooxygenases (COX).
Eicosatetraynoic acid is a lipoxygenase and cyclooxygenase inhibitor.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
A S Taylor et al.
Prostaglandins, 29(3), 449-458 (1985-03-01)
5,8,11,14-eicosatetraynoic acid (ETYA), a widely used inhibitor of cyclooxygenase and lipoxygenase, inhibited the incorporation of 14C-arachidonic acid into cell lipids of the murine thymoma EL4 whereas oleic acid had no effect. Inhibition appeared to result from the ability of ETYA
Günther F E Scherer et al.
FEBS letters, 581(22), 4205-4211 (2007-08-19)
Auxin increases phospholipase A(2) activity within 2min (Paul, R., Holk, A. and Scherer, G.F.E. (1998) Fatty acids and lysophospholipids as potential second messengers in auxin action. Rapid activation of phospholipase A(2) activity by auxin in suspension-cultured parsley and soybean cells.
Parissa Taheri et al.
Journal of plant physiology, 167(3), 201-208 (2009-09-05)
Vitamins are plant growth regulators and activators of defense responses against pathogens. The cytomolecular mechanisms involved in the induction of resistance by chemicals especially vitamins on monocotyledonous plants are largely unknown. Here, we show that riboflavin, which acts as a
Miriam Guizy et al.
American journal of physiology. Cell physiology, 289(5), C1251-C1260 (2005-07-01)
Dietary polyunsaturated fatty acids (PUFAs) have been reported to exhibit antiarrhythmic properties, which have been attributed to their availability to modulate Na(+), Ca(2+), and several K(+) channels. However, their effects on human ether-a-go-go-related gene (HERG) channels are unknown. In this
Kazuhiro Tamura et al.
Vascular pharmacology, 44(6), 411-416 (2006-05-03)
To address the role of prostaglandin E2 (PGE2) in tube formation of endothelial cells and the relationships between the action of PGE2 and vascular endothelial growth factor (VEGF), cultured human umbilical vein endothelial cells (HUVECs) were used to evaluate tube
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门