所有图片(2)
About This Item
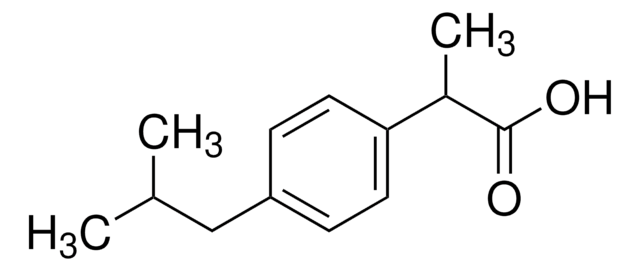
经验公式(希尔记法):
C17H21NO3
CAS号:
分子量:
287.35
MDL號碼:
分類程式碼代碼:
12352200
PubChem物質ID:
NACRES:
NA.77
推荐产品
品質等級
起源
Wyeth
儲存溫度
2-8°C
SMILES 字串
CCc1cccc2c3CCOC(CC)(CC(O)=O)c3[nH]c12
InChI
1S/C17H21NO3/c1-3-11-6-5-7-12-13-8-9-21-17(4-2,10-14(19)20)16(13)18-15(11)12/h5-7,18H,3-4,8-10H2,1-2H3,(H,19,20)
InChI 密鑰
NNYBQONXHNTVIJ-UHFFFAOYSA-N
基因資訊
human ... PTGS1(5742) , PTGS2(5743)
正在寻找类似产品? 访问 产品对比指南
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
其他客户在看
J A Balfour et al.
Drugs, 42(2), 274-299 (1991-08-01)
Etodolac is a nonsteroidal anti-inflammatory drug (NSAID) effective in the treatment of rheumatoid arthritis, osteoarthritis and ankylosing spondylitis, and in the alleviation of postoperative pain. Etodolac also provides relief of other types of pain, including that arising from gouty conditions
D C Brater et al.
Clinical rheumatology, 8 Suppl 1, 25-35 (1989-03-01)
The pharmacokinetics of etodolac, a new nonsteroidal anti-inflammatory drug, were compared in normal subjects, in patients with renal and hepatic disease, and in elderly patients. In 28 normal subjects, orally administered etodolac was rapidly absorbed. By 1.2 hours after ingestion
Shravan Kumar Tirunagari et al.
The Cochrane database of systematic reviews, (3)(3), CD007357-CD007357 (2009-07-10)
Etodolac is a selective cyclo-oxygenase-2 (COX-2) inhibitor, with evidence of efficacy in osteoarthritis and rheumatoid arthritis. Its analgesic efficacy in postoperative pain has not been clearly established. There are no systematic reviews on Etodolac's use in this condition. To assess
O Hazut et al.
International journal of clinical pharmacology and therapeutics, 49(9), 545-554 (2011-09-06)
COX inhibitors and β-adrenergic blockers were recently shown to reduce cancer progression in animal models through various mechanisms. These include the prevention of immune suppression during the critical perioperative period, and the preclusion of direct promoting effects of catecholamines and
N Zvaifler
Clinical rheumatology, 8 Suppl 1, 43-53 (1989-03-01)
Etodolac (Lodine, Ramodar, Ultradol), an anti-inflammatory, analgesic agent, is the first of a new class of nonsteroidal anti-inflammatory drugs (NSAIDs), the pyranocarboxylic acids. A review of the literature on numerous clinical studies showed that etodolac (200 to 600 mg/day) is
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门