所有图片(3)
About This Item
经验公式(希尔记法):
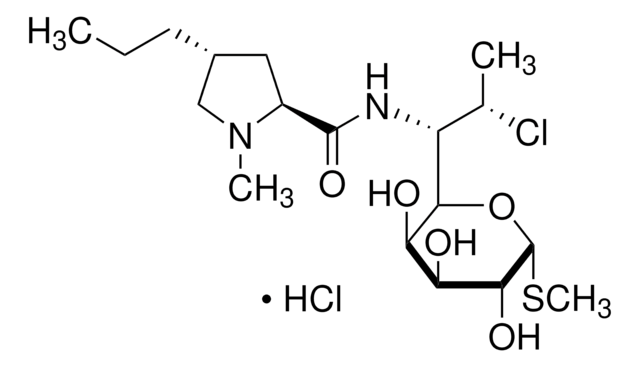
C18H33ClN2O5S · HCl
CAS号:
分子量:
461.44
Beilstein:
4070786
MDL號碼:
分類程式碼代碼:
51102829
PubChem物質ID:
NACRES:
NA.85
推荐产品
品質等級
形狀
powder or crystals
雜質
≤13%
溶解度
H2O: 50 mg/mL
抗生素活性譜
Gram-negative bacteria
Gram-positive bacteria
作用方式
protein synthesis | interferes
儲存溫度
2-8°C
SMILES 字串
Cl.CCC[C@@H]1C[C@H](N(C)C1)C(=O)N[C@H]([C@H](C)Cl)C2O[C@H](SC)[C@H](O)[C@@H](O)[C@H]2O
InChI
1S/C18H33ClN2O5S.ClH/c1-5-6-10-7-11(21(3)8-10)17(25)20-12(9(2)19)16-14(23)13(22)15(24)18(26-16)27-4;/h9-16,18,22-24H,5-8H2,1-4H3,(H,20,25);1H/t9-,10+,11-,12+,13-,14+,15+,16+,18+;/m0./s1
InChI 密鑰
AUODDLQVRAJAJM-XJQDNNTCSA-N
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
化学结构:大环内酯
應用
克林霉素用于研究细菌感染,例如 B 组链球菌病、细菌抗性和血浆蛋白结合。
用于研究细菌蛋白质合成。
生化/生理作用
克林霉素是一种由林可霉素制备的半合成林可酰胺类抗生素。 它通过与 50S 核糖体亚基的 23S rRNA 组分的氢键相互作用,抑制细菌蛋白质合成,从而诱导肽基-t-RNA 复合物的解离。它具有抗革兰氏阳性球菌的抗菌活性,以及抗弓形虫的抗原虫活性。
克林霉素盐酸盐对厌氧菌非常有效。
其他說明
林可酰胺类的抗菌和抗原生动物抗生素。
保持容器密闭,置于干燥通风处。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Lact. - Skin Sens. 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Sonja Löfmark et al.
The Journal of antimicrobial chemotherapy, 58(6), 1160-1167 (2006-10-19)
The aim was to study the long-term consequences of 1 week clindamycin administration regarding selection and persistence of resistance, resistance determinants and diversity of the Bacteroides spp. in the intestinal microflora. A total of 1306 Bacteroides isolates were collected from
Anouk E Muller et al.
Antimicrobial agents and chemotherapy, 54(5), 2175-2181 (2010-02-24)
The study presented here was performed to determine the pharmacokinetics of intravenously administered clindamycin in pregnant women. Seven pregnant women treated with clindamycin were recruited. Maternal blood and arterial and venous umbilical cord blood samples were obtained. Maternal clindamycin concentrations
A Burian et al.
The Journal of antimicrobial chemotherapy, 66(1), 134-137 (2010-11-04)
although plasma protein binding (PPB) is accepted to be an essential factor in reducing antimicrobial activity, little is known about the underlying mechanisms. One possibility includes impaired penetration of an antimicrobial into bacterial cells in the presence of PPB. As
Michael C Jewett et al.
Molecular systems biology, 9, 678-678 (2013-06-27)
Purely in vitro ribosome synthesis could provide a critical step towards unraveling the systems biology of ribosome biogenesis, constructing minimal cells from defined components, and engineering ribosomes with new functions. Here, as an initial step towards this goal, we report
J Spízek et al.
Applied microbiology and biotechnology, 64(4), 455-464 (2004-02-06)
Lincomycin and clindamycin are lincosamide antibiotics used in clinical practice. Both antibiotics are bacteriostatic and inhibit protein synthesis in sensitive bacteria. They may even be bactericidal at the higher concentrations that can be reached in vivo. Clindamycin is usually more
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门