B2134
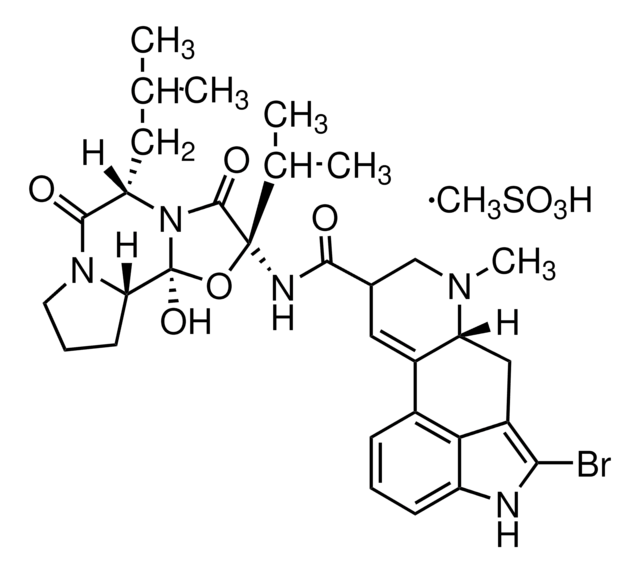
2-Bromo-α-ergocryptine methanesulfonate salt
solid
别名:
(+)-2-Bromo-12′-hydroxy-2′-(1-methylethyl)-5′-(2-methylpropyl)ergotaman-3′,6′-18-trione methanesulfonate salt, (+)-Bromocriptine methanesulfonate salt, Bromocriptine mesylate salt
About This Item
推荐产品
形狀
solid
光學活性
[α]20/D +95°, c = 1 in methanol: methylene chloride (1:1)(lit.)
顏色
white
溶解度
H2O: 0.8 mg/mL
ethanol: 23 mg/mL
儲存溫度
2-8°C
SMILES 字串
CS(O)(=O)=O.[H][C@@]12Cc3c(Br)[nH]c4cccc(C1=CC(CN2C)C(=O)N[C@@]5(O[C@]6(O)N([C@@H](CC(C)C)C(=O)N7CCC[C@@]67[H])C5=O)C(C)C)c34
InChI
1S/C32H40BrN5O5.CH4O3S/c1-16(2)12-24-29(40)37-11-7-10-25(37)32(42)38(24)30(41)31(43-32,17(3)4)35-28(39)18-13-20-19-8-6-9-22-26(19)21(27(33)34-22)14-23(20)36(5)15-18;1-5(2,3)4/h6,8-9,13,16-18,23-25,34,42H,7,10-12,14-15H2,1-5H3,(H,35,39);1H3,(H,2,3,4)/t18?,23-,24+,25+,31-,32+;/m1./s1
InChI 密鑰
NOJMTMIRQRDZMT-NEKRQHSLSA-N
基因資訊
human ... DRD2(1813) , DRD3(1814) , PRL(5617)
正在寻找类似产品? 访问 产品对比指南
應用
- as D2 agonist in bird zebra finches
- for Prl secretion inhibitor in mice
- for the inhibition of motility in planaria worm
生化/生理作用
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门