推荐产品
形狀
solid
顏色
yellow
溶解度
DMSO: >50 mg/mL
H2O: insoluble
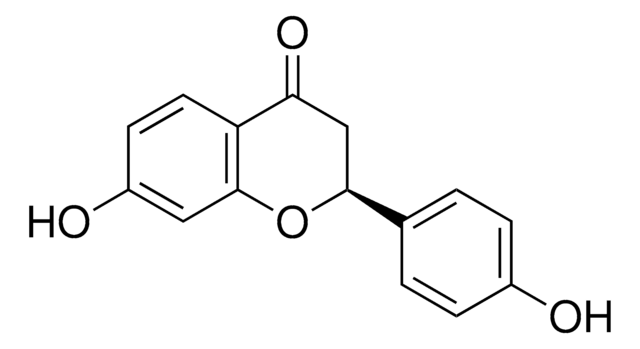
SMILES 字串
OC1=CC(O)=C(C(/C=C/C2=CC(O)=C(O)C=C2)=O)C=C1
InChI
1S/C15H12O5/c16-10-3-4-11(14(19)8-10)12(17)5-1-9-2-6-13(18)15(20)7-9/h1-8,16,18-20H/b5-1+
InChI 密鑰
AYMYWHCQALZEGT-ORCRQEGFSA-N
基因資訊
rat ... Alox5(25290)
正在寻找类似产品? 访问 产品对比指南
一般說明
Butein (2′,3,4,4′-tetrahydroxychalcone) is a chalcone and a flavonoid, that is produced in plants. It is a plant polyphenol and a bioactive constituent, that is extracted from the heartwood of Dalbergia odorifera, Caragana jubata and Rhus verniciflua stokes, and the stem bark of cashews (Semecarpus anacardium).
生化/生理作用
Butein exhibit several pharmacological activities, such as anti-oxidant and anti-inflammatory activity. It stimulates apoptotic cell death of human cervical cancer cells. It has therapeutic potentials for chronic diseases, including liver tuberculosis, obesity, diabetes and hypertension. Butein can repress migration and invasion of bladder, breast and pancreatic cancer cells.
Inhibits EGFR and Src tyrosine kinase activities; inhibits cAMP-dependent PDE-IV. Induces apoptosis in B16 melanoma cells and HL-60 human leukemia cells.
特點和優勢
This compound is a featured product for Kinase Phosphatase Biology research. Click here to discover more featured Kinase Phosphatase Biology products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
注意
Photosensitive
儲存類別代碼
13 - Non Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Dong-Oh Moon et al.
Toxicology in vitro : an international journal published in association with BIBRA, 24(7), 1927-1934 (2010-08-11)
3,4,2',4'-Tetrahydroxychalcone (butein) has potent anti-inflammatory, anti-cancer and anti-fibrogenic effects. However, little is known about the mechanism by which butein inhibits metastasis and invasion. This study aimed to investigate the effects of butein on the expression of matrix metalloproteinase (MMP-9) and
Butein induces apoptotic cell death of human cervical cancer cells
Yang P Y, et al.
Oncology Letters, 16(5), 6615-6623 (2018)
Agnieszka Szuster-Ciesielska et al.
Journal of gastroenterology, 48(2), 222-237 (2012-06-23)
Butein has been reported to prevent and partly reverse liver fibrosis in vivo; however, the mechanisms of its action are poorly understood. We, therefore, aimed to determine the antifibrotic potential of butein. We assessed the influence of the incubation of
Karin Schlangen et al.
Journal of experimental botany, 61(12), 3451-3459 (2010-06-23)
A chalcone 3-hydroxylase (CH3H) cDNA clone was isolated and characterized from Cosmos sulphureus petals accumulating butein (2',3,4,4'-tetrahydroxychalcone) derivatives as yellow flower pigments. The recombinant protein catalyses the introduction of an additional hydroxyl group in the B-ring of chalcones, a reaction
S M Yu et al.
European journal of pharmacology, 280(1), 69-77 (1995-06-23)
Butein, isolated from Dalbergia odorifera T. Chen, caused endothelium-dependent relaxation of rat aorta precontracted with phenylephrine. This effect was abolished in endothelium-denuded aorta and in endothelium-intact aorta in the presence of NG-monomethyl-L-arginine, oxyhemoglobin and methylene blue, whereas the effect was
商品
Epigenetic modifications are thought to occur through two key interconnected processes—DNA methylation and the covalent modification of histones.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门