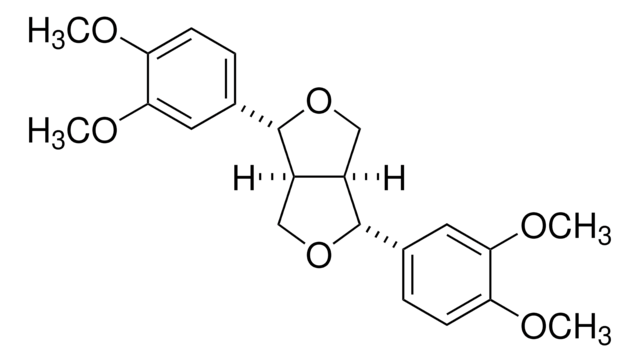
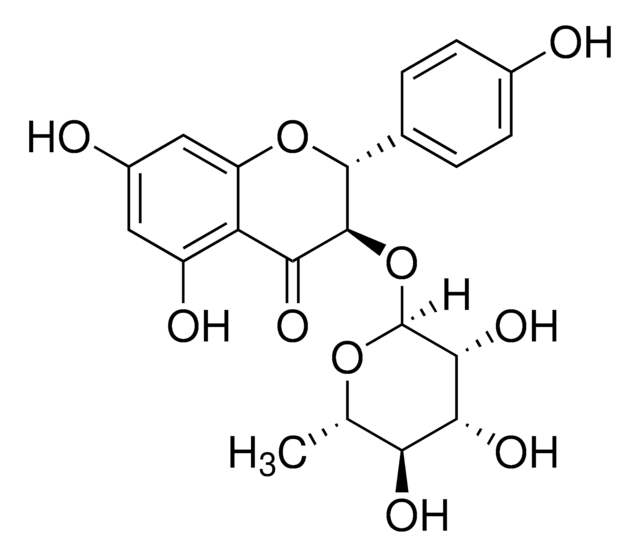
推荐产品
化驗
≥98%
形狀
powder
溶解度
H2O: 1 mg/mL, clear, colorless
應用
metabolomics
vitamins, nutraceuticals, and natural products
儲存溫度
2-8°C
SMILES 字串
O=C1C2=C(O)C=C(O)C=C2O[C@H](C3=CC(O)=C(O)C=C3)[C@H]1O[C@@]4([H])[C@H](O)[C@H](O)[C@@H](O)[C@H](C)O4
InChI
1S/C21H22O11/c1-7-15(26)17(28)18(29)21(30-7)32-20-16(27)14-12(25)5-9(22)6-13(14)31-19(20)8-2-3-10(23)11(24)4-8/h2-7,15,17-26,28-29H,1H3/t7-,15-,17+,18+,19-,20+,21-/m0/s1
InChI 密鑰
ZROGCCBNZBKLEL-MFSALPCASA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Astilbin belongs to the class of dihydroflavanol compounds and is the chief biologically active component of Rhizoma Smilacis Glabrae (RSG) extract. RSG is obtained from the dried rhizome of Smilax Glabra Roxb.
應用
Astilbin from Engelhardtia roxburghiana, a flavonoid phytochemical with antibacterial activity, may be used to study its pharmacology and the effects of its antiproliferative activities versus a variety of cell types.
生化/生理作用
Astilbin exerts various biological activities such as anti-oxidative, anti-bacterial, and inhibition of contact hypersensitivity. Also, it shows insecticidal, anti-oedematogenic, antinociceptive and antidepressant properties. It has an inhibitory effect on the aldose reductase enzyme, the accumulation of sorbitol, and a protective effect on the liver. Astilbin is helpful against autoimmune diseases such as rheumatoid arthritis due to its ability to reduce matrix metalloproteinase (MMP) activity and Nitric oxide production in T lymphocytes.
Flavonoid phytochemical found in St. John′s wort and Traditional Chinese Medicine herbal preparations. Immunosuppresive. Antiproliferative.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Xiao-Ya Shang et al.
Journal of Asian natural products research, 14(10), 966-972 (2012-10-11)
A new dihydroflavonol glycoside dimer 6,6-bisastilbin (1) and a new nitrile-containing metabolite (Z)-5α,6β-dihydroxy-4β-methoxy-2-cyclohexene-Δ(1,α)-acetonitrile (2), together with three known analogs, bauhinin, bauhinilide, and dehydrodicatechin A, have been isolated from an ethanol extract of Bauhinia aurea. Their structures were determined by spectroscopic
Fernando Petacci et al.
Biological research, 43(1), 63-74 (2010-12-16)
Astilbin (5,7,3',4'-tetrahydroxy-2,3-dihydroflavonol-3-ß-o-rhamnoside), a flavonoid with a large range of biological activities, was isolated from Dimorphandra mollis, a shrub common to the Brazilian Cerrado. The purpose of this study is to verify the effects of astilbin on myeloperoxidase (MPO) and horseradish
Lvyi Chen et al.
Planta medica, 77(16), 1769-1773 (2011-05-27)
Astilbin is a flavonoid compound isolated from the rhizome of Smilax china L. The effects and possible mechanisms of astilbin on hyperuricemia and nephropathy rats were elucidated in this study. Different dosages of astilbin (1.25, 2.5, and 5.0 mg/kg) were
Nicolas Landrault et al.
Journal of agricultural and food chemistry, 50(7), 2046-2052 (2002-03-21)
Phenolics from grapes and wines can play a role against oxidation and development of atherosclerosis. Stilbenes have been shown to have cancer chemopreventive activity and to protect lipoproteins from oxidative damage. A method for the direct determination of stilbene oligomers
Yong-Jiang Xu et al.
Planta medica, 77(11), 1139-1148 (2011-02-18)
The extracts of two medicinal plants used in traditionalmedicine against malariawere characterized by means of an LC‑SPE‑NMR and LC‑MS platform. The structure of a series of major constituents from Bafodeya benna, as well as minor constituents from Ormocarpum kirkii, was
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门