所有图片(1)
About This Item
经验公式(希尔记法):
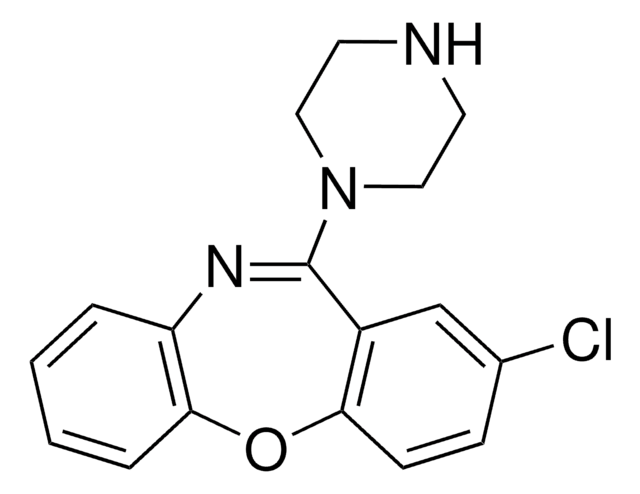
C17H16ClN3O
CAS号:
分子量:
313.78
EC號碼:
MDL號碼:
分類程式碼代碼:
12352200
PubChem物質ID:
NACRES:
NA.77
推荐产品
形狀
powder
溶解度
methanol: soluble
起源
Wyeth
SMILES 字串
Clc1ccc2Oc3ccccc3N=C(N4CCNCC4)c2c1
InChI
1S/C17H16ClN3O/c18-12-5-6-15-13(11-12)17(21-9-7-19-8-10-21)20-14-3-1-2-4-16(14)22-15/h1-6,11,19H,7-10H2
InChI 密鑰
QWGDMFLQWFTERH-UHFFFAOYSA-N
基因資訊
human ... DRD1(1812) , DRD2(1813) , DRD3(1814) , DRD4(1815) , DRD5(1816) , HTR2A(3356) , HTR2B(3357) , HTR2C(3358) , SLC6A2(6530) , SLC6A4(6532)
正在寻找类似产品? 访问 产品对比指南
應用
Amoxapine has been used as an antidepressant drug to test its effect on locomotion and egg release in response to gain-of-functional mutations in potassium (K+) channels (unc-58) of C. elegans. It has also been used as an antipsychotic drug to test its effect on the viability of glioblastoma cells.
生化/生理作用
Amoxapine, a structural analog of clozapine, is a human ether a-go-go (hERG) channel blocker. It is also an N-methylated metabolite of loxapine. It is a tricyclic antidepressant that inhibits the uptake of norepinephrine and blocks 5- hydroxytryptamine (HT2 ) serotonergic receptors.
特點和優勢
This compound is featured on the Biogenic Amine Transporters page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
This compound was developed by Wyeth. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
S G Jue et al.
Drugs, 24(1), 1-23 (1982-07-01)
Amoxapine is an N-demethylated dibenzoxazepine closely related in the neuroleptic loxapine. Its tricyclic structure appears to give it antidepressant properties resembling imipramine and amitriptyline. In uncontrolled trials it was shown to have antidepressant activity in usual doses up to 200
S Kapur et al.
Biological psychiatry, 45(9), 1217-1220 (1999-05-20)
All currently available atypical antipsychotics have, at clinically relevant doses: i) high serotonin (5-HT)2 occupancy; ii) greater 5-HT2 than dopamine (D)2 occupancy; and iii) a higher incidence of extrapyramidal side effects when their D2 occupancy exceeds 80%. A review of
Imran B Chaudhry et al.
Journal of clinical psychopharmacology, 27(6), 575-581 (2007-11-16)
It has been proposed that the lack of extrapyramidal side effects of atypical antipsychotic drugs is caused by their fast dissociation or low affinity for the D2 receptor or their concomitant high affinity for other receptors, for example, 5HT2 and
Yan-Lin He et al.
The Journal of pharmacology and experimental therapeutics, 332(2), 437-445 (2009-11-17)
Ion channels are known to be modulated by antidepressant drugs, but the molecular mechanisms are not known. We have shown that the antidepressant drug amoxapine suppresses rectifier outward K(+) (I(K)) currents in mouse cortical neurons. At a concentration of 10
F Jenck et al.
Progress in neuro-psychopharmacology & biological psychiatry, 18(3), 563-574 (1994-05-01)
A variety of antidepressants of different chemical classes were tested for their in vivo and in vitro activity at 5-HT1C receptors in the brain. Conventional tricyclic antidepressants (imipramine, desipramine, maprotiline, clomipramine, trimipramine, amitriptyline, nortriptyline, doxepin, amoxapine, oxaprotiline) and two atypical
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门