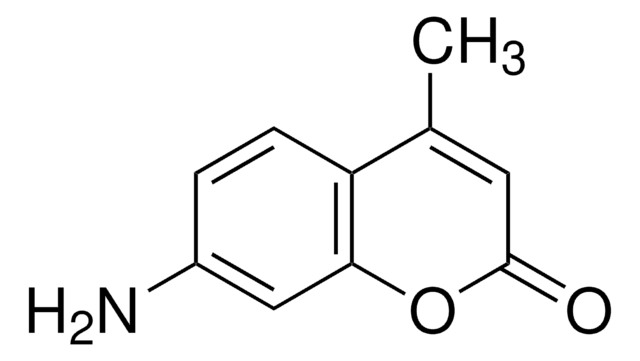
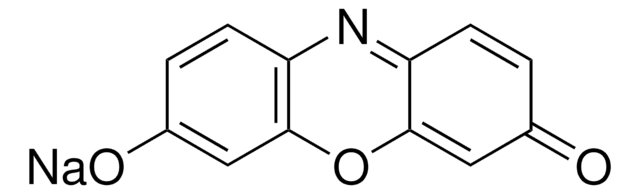
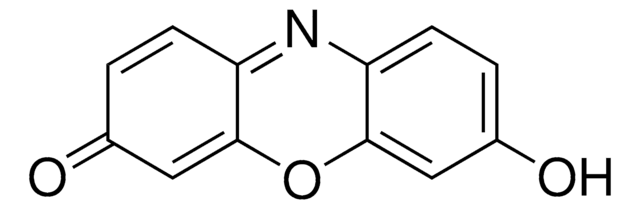
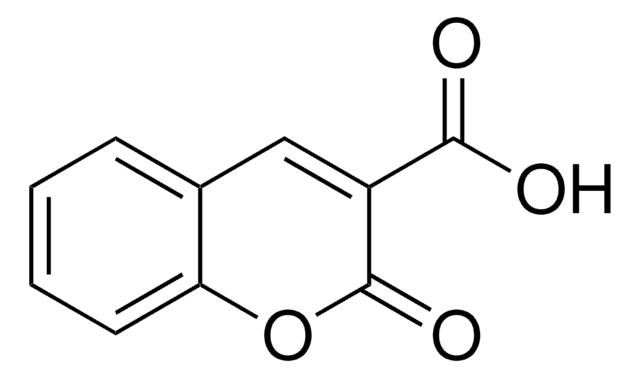
推荐产品
產品線
BioReagent
化驗
≥98.0% (TLC)
形狀
powder
mp
≥250 °C (lit.)
溶解度
DMF: soluble
alcohols: soluble
螢光
λex 408 nm; λem 450 nm in methanol
適合性
suitable for fluorescence
SMILES 字串
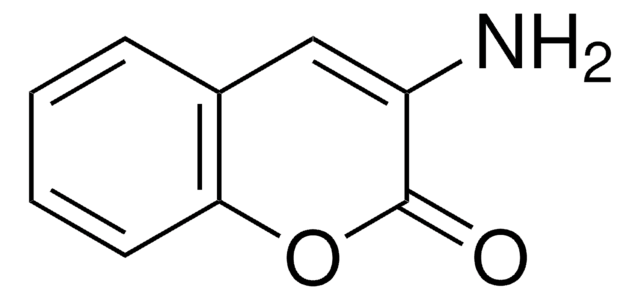
Oc1ccc2C=C(C#N)C(=O)Oc2c1
InChI
1S/C10H5NO3/c11-5-7-3-6-1-2-8(12)4-9(6)14-10(7)13/h1-4,12H
InChI 密鑰
IJQYTHQDUDCJEQ-UHFFFAOYSA-N
基因資訊
human ... MIF(4282)
應用
3-Cyano-7-hydroxycoumarin is closely related to 3-cyano-7-ethoxycoumarin which is used as a fluorometric substrate and inhibitor for cytochrome P-450 enzymes and cytochrome P-450-dependent mixed function oxidases. 3-Cyano-7-hydroxycoumarin may be useful to study the kinetics and substrate specificity of cytochrome P-450s.
包裝
Bottomless glass bottle. Contents are inside inserted fused cone.
訊號詞
Warning
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Jay J Agarwal et al.
PloS one, 8(5), e63028-e63028 (2013-05-15)
TRAM-34, a clotrimazole analog characterized as a potent and selective inhibitor of intermediate-conductance, calcium-activated K(+) (IKCa) channels, has been used extensively in vitro and in vivo to study the biological roles of these channels. The major advantage of TRAM-34 over
Joonyoung F Joung et al.
Physical chemistry chemical physics : PCCP, 19(37), 25509-25517 (2017-09-14)
Proton dissociation (PD) reactions of weak acids and proton transfer (PT) processes in aqueous solutions are strongly influenced by ions. However, a detailed molecular picture that describes how ions affect the rates of PD and PT processes is still missing.
Ilana Berger et al.
PloS one, 6(11), e26794-e26794 (2011-11-10)
Cytosolic sulfotransferases (SULTs) are mammalian enzymes that detoxify a wide variety of chemicals through the addition of a sulfate group. Despite extensive research, the molecular basis for the broad specificity of SULTs is still not understood. Here, structural, protein engineering
Samuel Koenig et al.
Aquatic toxicology (Amsterdam, Netherlands), 108, 11-17 (2011-11-19)
Variations in cytochrome P450 enzyme (CYPs) distribution and function between animal groups could result in differential metabolism and elimination kinetics for certain contaminants. Although a number of studies have suggested that differences in polychlorobiphenyl (PCB) accumulation profiles between crustacea and
Corinna Krempl et al.
Insect biochemistry and molecular biology, 78, 69-77 (2016-10-18)
Gossypol is a polyphenolic secondary metabolite produced by cotton plants, which is toxic to many organisms. Gossypol's aldehyde groups are especially reactive, forming Schiff bases with amino acids of proteins and cross-linking them, inhibiting enzyme activities and contributing to toxicity.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门