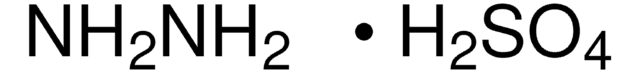推荐产品
product name
二乙醚, puriss., contains ~5 mg/L 2,6-di-tert.-butyl-4-methylphenol as stabilizer, meets analytical specification of Ph. Eur., BP, ≥99.5% (GC)
蒸汽密度
2.6 (vs air)
品質等級
等級
puriss.
化驗
≥99.5% (GC)
形狀
liquid
自燃溫度
320 °F
包含
~5 mg/L 2,6-di-tert.-butyl-4-methylphenol as stabilizer
expl. lim.
36.5 %
雜質
acidic reac. Impurities, in accordance
aldehydes, in accordance
residual solvents, in accordance
≤0.0002% peroxides (as H2O2)
≤0.002% free acid (as CH3COOH)
≤0.002% non-volatile matter
≤0.2% water (Karl Fischer)
折射率
n20/D 1.3530 (lit.)
bp
34.6 °C (lit.)
mp
−116 °C (lit.)
密度
0.706 g/mL at 25 °C (lit.)
SMILES 字串
CCOCC
InChI
1S/C4H10O/c1-3-5-4-2/h3-4H2,1-2H3
InChI 密鑰
RTZKZFJDLAIYFH-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
其他說明
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 4 Oral - Flam. Liq. 1 - STOT SE 3
標靶器官
Central nervous system
安全危害
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 1
閃點(°F)
-40.0 °F - closed cup
閃點(°C)
-40 °C - closed cup
其他客户在看
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门