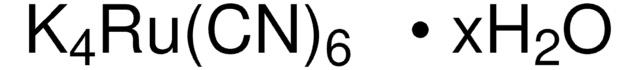所有图片(2)
About This Item
线性分子式:
K3Co(CN)6
CAS号:
分子量:
332.33
EC號碼:
MDL號碼:
分類程式碼代碼:
12352202
PubChem物質ID:
NACRES:
NA.55
推荐产品
等級
for analytical purposes
化驗
≥97.0%
形狀
crystalline
反應適用性
reagent type: catalyst
core: cobalt
雜質
≤0.1% free cyanide
密度
1.878 g/mL at 25 °C (lit.)
負離子痕跡
sulfate (SO42-): ≤500 mg/kg
正離子痕跡
Fe: ≤50 mg/kg
適合性
suitable for acidity or alkalinity (alkalinity <= 0.15 meq/g)
SMILES 字串
[K+].[K+].[K+].N#C[Co-3](C#N)(C#N)(C#N)(C#N)C#N
InChI
1S/6CN.Co.3K/c6*1-2;;;;/q;;;;;;-3;3*+1
InChI 密鑰
VSUFNKULKBVQQW-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
六氰钴酸钾(III)可用作络合剂以合成双金属氰化催化剂:
- 用于羰基化合物与芳香胺的化学选择性还原胺化,以聚甲基氢硅氧烷为还原剂合成取代胺。
- 环氧丙烷的开环聚合,以合成多元醇。
- CO2 与含水环氧化物的偶联反应,以合成环状碳酸盐。
儲存類別代碼
13 - Non Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Edward E Little et al.
Environmental science and pollution research international, 14(5), 333-337 (2007-08-29)
Cobalt cyanide complexes often result when ore is treated with cyanide solutions to extract gold and other metals. These have recently been discovered in low but significant concentrations in effluents from gold leach operations. This study was conducted to determine
A 59Co NMR relaxation probe of macromolecule anionic binding sites.
T Raj et al.
Analytical biochemistry, 106(2), 373-376 (1980-08-01)
Reynhardt EC and Boeyens JCA.
Acta Crystallographica Section B, Structural Science, Crystal Engineering and Materials, 28(2), 524-529 (1972)
Effect of pH on Retention of 134Cs and 60Co from MAW by Potassium Hexacyanocobaltate(III).
Mekhail FM, et al.
Isotopes in Environmental and Health Studies, 130-133 (1991)
Xin Zhang et al.
ChemSusChem, 14(1), 467-478 (2020-10-13)
Electrolytic water splitting using surplus electricity represents one of the most cost-effective and promising strategies for hydrogen production. The high overpotential of the oxygen-evolution reaction (OER) caused by the multi-electron transfer process with a high chemical energy barrier, however, limits
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门