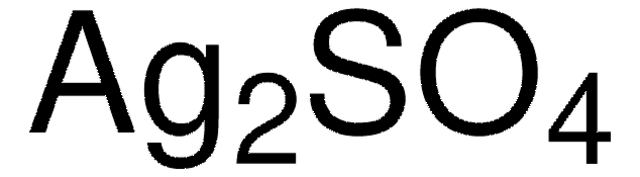所有图片(1)
About This Item
线性分子式:
Ag2SO4
CAS号:
分子量:
311.80
EC號碼:
MDL號碼:
分類程式碼代碼:
12352302
PubChem物質ID:
NACRES:
NA.21
推荐产品
等級
puriss.
化驗
≥99%
形狀
powder or crystals
反應適用性
reagent type: catalyst
core: silver
mp
652 °C (lit.)
負離子痕跡
chloride (Cl-): ≤10 mg/kg
nitrate (NO3-): ≤100 mg/kg
正離子痕跡
Cu: ≤50 mg/kg
Fe: ≤50 mg/kg
Pb: ≤50 mg/kg
SMILES 字串
[Ag+].[Ag+].[O-]S([O-])(=O)=O
InChI
1S/2Ag.H2O4S/c;;1-5(2,3)4/h;;(H2,1,2,3,4)/q2*+1;/p-2
InChI 密鑰
YPNVIBVEFVRZPJ-UHFFFAOYSA-L
正在寻找类似产品? 访问 产品对比指南
一般說明
Silver sulfate (Ag2SO4) is the disilver(I) salt of sulfuric acid. It participates in the TiO2-assisted photooxidation of substituted benzyltrimethylsilanes in acetonitrile. Diarylethanes were obtained in 50-74% yields. It has been reported as one of the common atmospheric silver corrosion product.
應用
Silver sulfate may be employed in the following studies:
- Iodination reagent in combination with iodine for the synthesis of iododerivatives.
- Synthesis of iodinated uredines.
- Preparation of silver sulfate reagent, which is required for the determination of Chemical Oxygen Demand (COD).
訊號詞
Danger
危險聲明
危險分類
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1
儲存類別代碼
13 - Non Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Chemical stability of plasmon-active silver tips for tip-enhanced Raman spectroscopy.
Kalbacova J, et al.
Nanospectroscopy, 1(1), 12-18 (2014)
Titanium dioxide photocatalysed oxidation of benzyltrimethylsilanes in the presence of silver sulfate.
Baciocchi E, et al.
Journal of the Chemical Society. Chemical Communications, 1, 59-60 (1992)
Ponnusamy Velusamy et al.
Journal of advanced research, 5(1), 19-25 (2015-02-17)
The photocatalytic decoloration of an organic dye, ethyl violet (EV), has been studied in the presence of TiO2 and the addition of β-Cyclodextrin (β-CD) with TiO2 (TiO2-β-CD) under UV-A light irradiation. The different operating parameters like initial concentration of dye
Iodination of aromatic amines with iodine and silver sulfate.
Sy W-W.
Synthetic Communications, 22(22), 3215-3219 (1992)
Iodination with Silver Sulfate and Iodine. II. Uridines.
Sy W-W.
Synthetic Communications, 20(21), 3391-3394 (1990)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门