Y0001210
Tobramycin for identification
European Pharmacopoeia (EP) Reference Standard
别名:
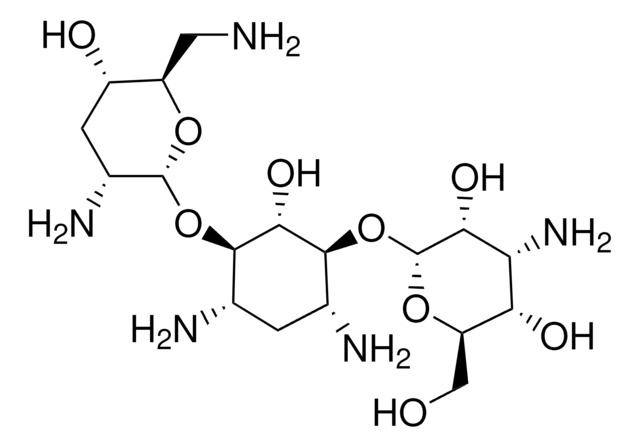
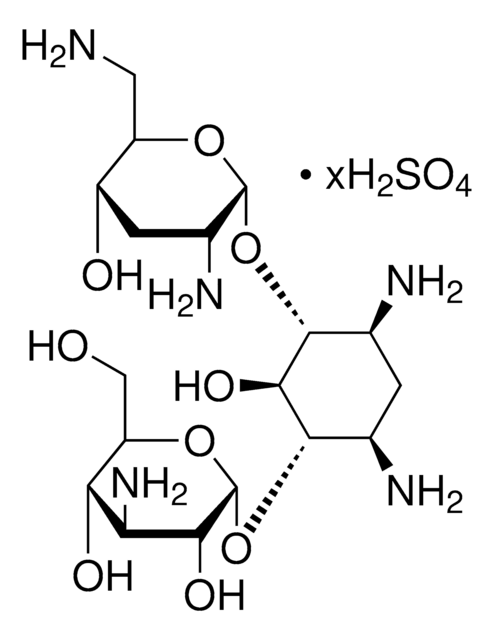
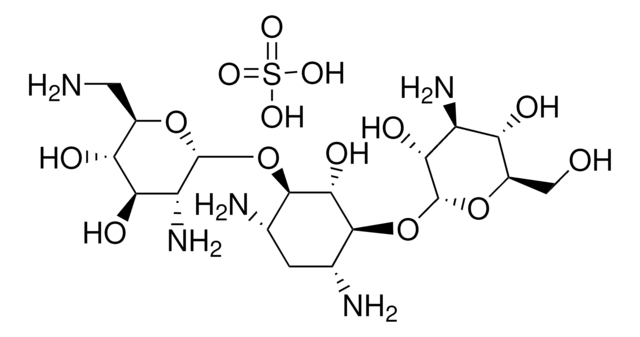
Tobramycin, Nebramycin Factor 6, O-[3-Amino-3-deoxy-α-D-glucopyranosyl-(1→6)]-O-[2,6-diamino-2,3,6-trideoxy-α-D-ribohexopyranosyl-(1→4)]-2-deoxy-D-streptamine
登录查看公司和协议定价
所有图片(1)
About This Item
经验公式(希尔记法):
C18H37N5O9
CAS号:
分子量:
467.51
Beilstein:
1357507
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推荐产品
等級
pharmaceutical primary standard
API 家族
tobramycin
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
SMILES 字串
NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O[C@H]3O[C@H](CO)[C@@H](O)[C@@H](N)[C@H]3O)[C@H]2O)[C@H](N)C[C@@H]1O
InChI
1S/C18H37N5O9/c19-3-9-8(25)2-7(22)17(29-9)31-15-5(20)1-6(21)16(14(15)28)32-18-13(27)11(23)12(26)10(4-24)30-18/h5-18,24-28H,1-4,19-23H2/t5-,6+,7+,8-,9+,10+,11+,12+,13+,14-,15+,16-,17+,18+/m0/s1
InChI 密鑰
NLVFBUXFDBBNBW-SNGYORCQSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.
For further information and support please go to the website of the issuing Pharmacopoeia.
For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Tobramycin for identification EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
Tobramycin is an aminoglycoside.
Mode of Action: Binds to 70S ribosomal subunit; inhibits translocation; elicits miscoding.
Spectrum of Activity: Gram negative bacteria. Not effective against Enterococci.
Mode of Action: Binds to 70S ribosomal subunit; inhibits translocation; elicits miscoding.
Spectrum of Activity: Gram negative bacteria. Not effective against Enterococci.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Warning
危險聲明
危險分類
Repr. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
Tobramycin inhalation powder (Tobi Podhaler) for cystic fibrosis.
The Medical letter on drugs and therapeutics, 55(1419), 51-52 (2013-06-26)
Kate McKeage
Drugs, 73(16), 1815-1827 (2013-11-07)
Inhaled tobramycin, an aminoglycoside antibacterial, has been in widespread use in the form of a nebulized solution against Pseudomonas aeruginosa for many years. More recently, tobramycin inhalation powder (TIP; TOBI(®) Podhaler(™)) was formulated using PulmoSphere(™) technology for administration as a
David E Geller et al.
Journal of aerosol medicine and pulmonary drug delivery, 24(4), 175-182 (2011-03-15)
Abstract At present, the only approved inhaled antipseudomonal antibiotics for chronic pulmonary infections in patients with cystic fibrosis (CF) are nebulized solutions. However, prolonged administration and cleaning times, high administration frequency, and cumbersome delivery technologies with nebulizers add to the
C L Bonsignore
Pediatric nursing, 24(3), 258-259 (1999-02-13)
Inhaled tobramycin was recently approved by the FDA in a 300 mg formulation for inhalation. The new product, manufactured by PathoGenesis Corporation, is referred to as TOBI and is indicated for cystic fibrosis patients with Pseudomonas aeruginosa. The advantage of
Susan M Cheer et al.
Drugs, 63(22), 2501-2520 (2003-11-12)
Specifically formulated for nebulisation using the PARI LC PLUS reusable nebuliser, tobramycin solution for inhalation (TSI) [TOBI] provides a high dose of tobramycin (an aminoglycoside antibacterial with good activity against Pseudomonas aeruginosa) to the lungs of patients with cystic fibrosis
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门