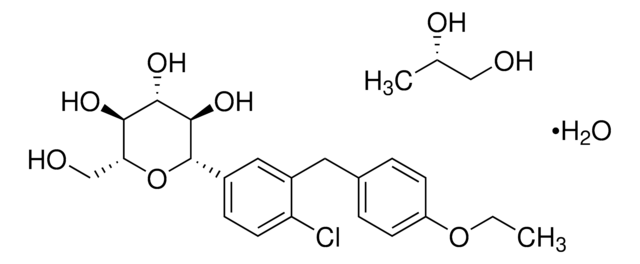
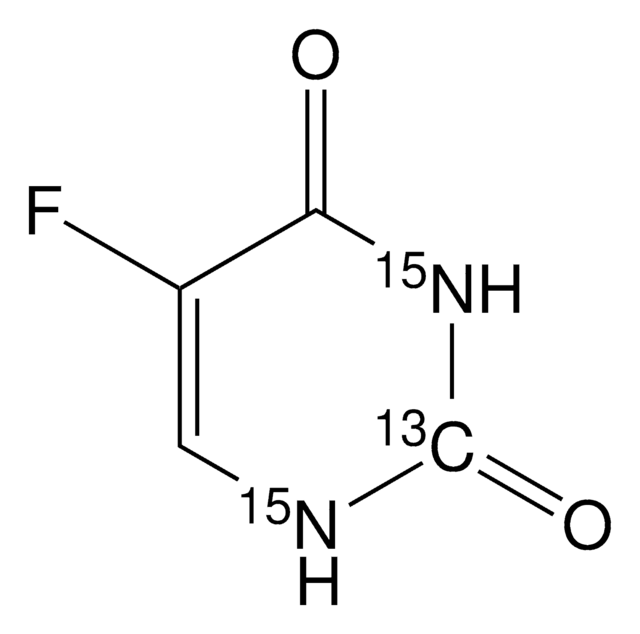
所有图片(1)
About This Item
经验公式(希尔记法):
C16H14F3N3O2S
CAS号:
分子量:
369.36
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推荐产品
等級
pharmaceutical primary standard
API 家族
lansoprazole
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
SMILES 字串
Cc1c(CS(=O)c2nc3ccccc3[nH]2)nccc1OCC(F)(F)F
InChI
1S/C16H14F3N3O2S/c1-10-13(20-7-6-14(10)24-9-16(17,18)19)8-25(23)15-21-11-4-2-3-5-12(11)22-15/h2-7H,8-9H2,1H3,(H,21,22)
InChI 密鑰
MJIHNNLFOKEZEW-UHFFFAOYSA-N
基因資訊
human ... ATP4A(495) , ATP4B(496)
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Lansoprazole for peak identification EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
产品编号
说明
价格
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Nahuai Badiola et al.
PloS one, 8(3), e58837-e58837 (2013-03-23)
A key event in the pathogenesis of Alzheimer's disease (AD) is the accumulation of amyloid-β (Aβ) species in the brain, derived from the sequential cleavage of the amyloid precursor protein (APP) by β- and γ-secretases. Based on a systems biology
Jyh-Ming Liou et al.
Lancet (London, England), 381(9862), 205-213 (2012-11-20)
Whether sequential treatment can replace triple therapy as the standard treatment for Helicobacter pylori infection is unknown. We compared the efficacy of sequential treatment for 10 days and 14 days with triple therapy for 14 days in first-line treatment. For
Jun-Won Chung et al.
Journal of gastroenterology and hepatology, 27(11), 1675-1680 (2012-08-02)
Increased resistance of Helicobacter pylori to antibiotics has increased the need to develop new first-line treatments for H. pylori. We have prospectively evaluated 10-day sequential versus conventional triple therapy in peptic ulcer patients. One hundred and fifty-nine patients with peptic ulcer
Hala H Mosli et al.
The Journal of clinical endocrinology and metabolism, 97(9), E1731-E1735 (2012-06-23)
Chromogranin A (CgA) is used as a generic tumor marker for neuroendocrine tumors. Proton pump inhibitors (PPI) are known to increase CgA, but it is not clear to what extent, and there is little information on how long PPI need
Grace Chai et al.
Pediatrics, 130(1), 23-31 (2012-06-20)
To describe trends in outpatient prescription drug utilization in US children and the changes in major areas of pediatric therapeutic use for the years 2002 through 2010. Large prescription databases (the IMS Vector One: National and Total Patient Tracker) were
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门