推荐产品
等級
pharmaceutical primary standard
API 家族
letrozole
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
SMILES 字串
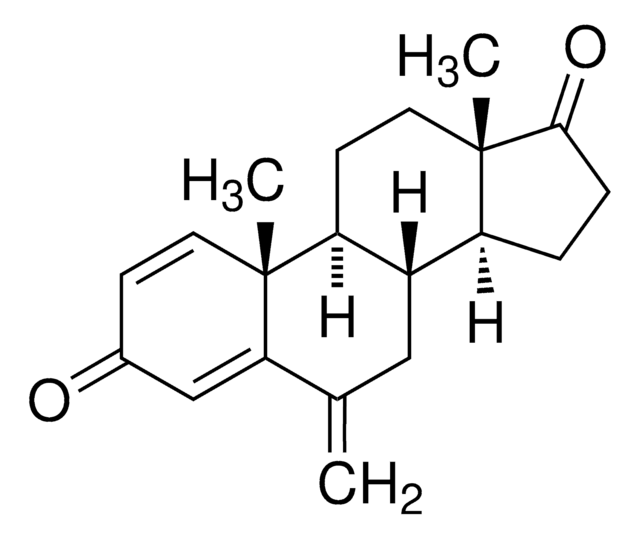
N#CC(C=C1)=CC=C1C(N2C=NC=N2)C3=CC=C(C#N)C=C3
InChI
1S/C17H11N5/c18-9-13-1-5-15(6-2-13)17(22-12-20-11-21-22)16-7-3-14(10-19)4-8-16/h1-8,11-12,17H
InChI 密鑰
HPJKCIUCZWXJDR-UHFFFAOYSA-N
基因資訊
human ... CYP19A1(1588)
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Letrozole EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
来曲唑是第三代非甾体芳香酶抑制剂。 它是芳香酶系统的竞争性抑制剂,因此可抑制雄激素向雌激素转化。来曲唑通过竞争性结合到该酶的细胞色素P450亚基的血红素上而抑制芳香酶,导致所有组织中雌激素的生物合成减少。
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
产品编号
说明
价格
訊號詞
Warning
危險聲明
危險分類
Repr. 2 - STOT RE 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
G Allevi et al.
British journal of cancer, 108(8), 1587-1592 (2013-04-13)
The objective of this study was to determine the optimal scheduling of 2.5 mg daily letrozole in neoadjuvant breast cancer patients to obtain pathological complete response (pathCR) and assess Ki-67 expression as an early predictor of response. This single institution
Pamela J Goodwin et al.
Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 32(21), 2231-2239 (2014-06-18)
Obesity is associated with poor outcomes in women with operable breast cancer. Lifestyle interventions (LIs) that help women reduce their weight may improve outcomes. We conducted a multicenter randomized trial comparing mail-based delivery of general health information alone or combined
Antonio C Wolff et al.
Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 31(2), 195-202 (2012-12-13)
Recent data showed improvement in progression-free survival (PFS) when adding everolimus to exemestane in patients with advanced breast cancer experiencing recurrence/progression after nonsteroidal aromatase inhibitor (AI) therapy. Here, we report clinical outcomes of combining the mammalian target of rapamycin (mTOR)
Valentina Guarneri et al.
Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 32(10), 1050-1057 (2014-03-05)
This is a randomized, double-blind, placebo-controlled study aimed to evaluate the clinical and biologic effects of letrozole plus lapatinib or placebo as neoadjuvant therapy in hormone receptor (HR) -positive/human epidermal growth factor receptor 2 (HER2) -negative operable breast cancer. Ninety-two
Israel Ortega et al.
Fertility and sterility, 99(3), 889-896 (2012-12-04)
To evaluate the effects of letrozole on ovarian size and steroidogenesis in vivo, as well as on proliferation and steroidogenesis of theca-interstitial cells alone and in coculture with granulosa cells using an in vitro model. In vivo and in vitro studies. Research laboratory. Immature
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门