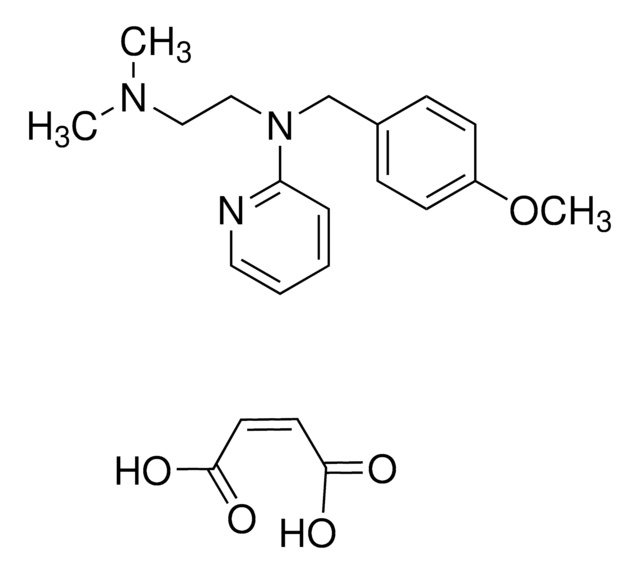
推荐产品
等級
pharmaceutical primary standard
API 家族
emedastine
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
InChI
1S/C17H26N4O.2C4H4O4/c1-3-22-14-13-21-16-8-5-4-7-15(16)18-17(21)20-10-6-9-19(2)11-12-20;2*5-3(6)1-2-4(7)8/h4-5,7-8H,3,6,9-14H2,1-2H3;2*1-2H,(H,5,6)(H,7,8)/b;2*2-1+
InChI 密鑰
FWLKKPKZQYVAFR-LVEZLNDCSA-N
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Emedastine difumarate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Ryoichi Morita et al.
Journal of controlled release : official journal of the Controlled Release Society, 90(1), 109-117 (2003-05-28)
In vitro dissolution tests of novel controlled release tablets, the poly(vinyl alcohol) (PVA) swelling controlled release system (SCRS), were performed by various methods under different conditions in the sinking state in water. The in vitro release profiles of various tests
Shoichi Harada et al.
Drug metabolism and pharmacokinetics, 20(5), 331-336 (2005-11-08)
In vivo percutaneous absorption of emedastine difumarate was investigated in rats and compared with rat skin in vitro. Since emedastine entering the systemic circulation is mostly excreted in bile, we first came up with the method of collecting bile with
Mark B Abelson et al.
Clinical therapeutics, 24(3), 445-456 (2002-04-16)
When selecting treatment for allergic conjunctivitis, a primary concern is whether to choose local or systemic therapy. This study compared the efficacy of topical emedastine 0.05% ophthalmic solution with that of oral loratadine 10 mg and their combination in the
M Eugenia Sanchis-Merino et al.
Experimental eye research, 86(5), 791-797 (2008-04-02)
In order to compare the relative efficacy of topical antihistamines with balanced saline solution (BSS) and benzalkonium chloride (BC) in the early phase of allergic conjunctivitis in an animal model of ocular anaphylaxis, 96 male guinea pigs were sensitized with
P Haicl et al.
Ceska a slovenska oftalmologie : casopis Ceske oftalmologicke spolecnosti a Slovenske oftalmologicke spolecnosti, 60(1), 58-63 (2004-03-12)
To determine, whether the immediately originated allergic reaction of conjunctivas may be treated with local H1 antihistaminic drugs in a monotherapy (Allergodil gtt, Emadine gtt). The group included 30 patients at the age of 17 to 40 years at the
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门