推荐产品
等級
pharmaceutical primary standard
API 家族
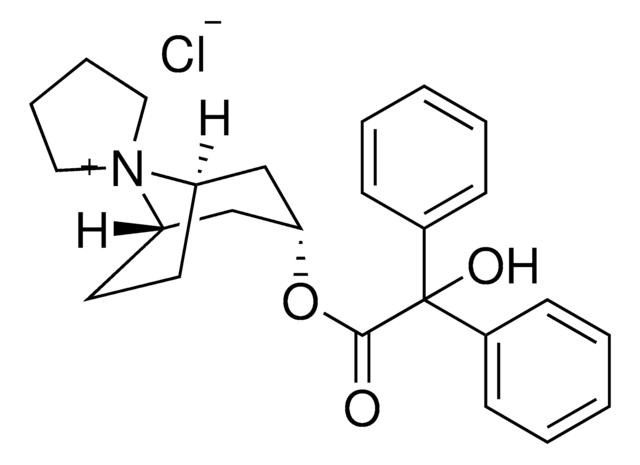
trospium
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
SMILES 字串
[Cl-].[N+]21(C3CCC2CC(C3)OC(=O)C(O)(c5ccccc5)c4ccccc4)CCCC1
InChI
1S/C25H30NO3.ClH/c27-24(25(28,19-9-3-1-4-10-19)20-11-5-2-6-12-20)29-23-17-21-13-14-22(18-23)26(21)15-7-8-16-26;/h1-6,9-12,21-23,28H,7-8,13-18H2;1H/q+1;/p-1
InChI 密鑰
RVCSYOQWLPPAOA-UHFFFAOYSA-M
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Trospium chloride EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
Anthony G Visco et al.
The New England journal of medicine, 367(19), 1803-1813 (2012-10-06)
Anticholinergic medications and onabotulinumtoxinA are used to treat urgency urinary incontinence, but data directly comparing the two types of therapy are needed. We performed a double-blind, double-placebo-controlled, randomized trial involving women with idiopathic urgency urinary incontinence who had five or
Scott A MacDiarmid et al.
Urology, 77(1), 24-29 (2010-10-26)
This study used pooled data from 2 large, phase III, double-blind, randomized, placebo-controlled studies for a subgroup analysis of the safety and efficacy of trospium chloride extended-release (XR) in men with overactive bladder (OAB). A subgroup analysis was performed on
Martina Urbanova et al.
Journal of pharmaceutical sciences, 102(4), 1235-1248 (2013-01-30)
Analysis of C cross-polarization magic angle spinning (CP/MAS) nuclear magnetic resonance (NMR), differential scanning calorimetry (DSC), Fourier transform infrared (FTIR), and X-ray powder diffraction data of trospium chloride (TCl) products crystallized from different mixtures of water-ethanol [φ(EtOH) = 0.5-1.0] at
R Zhang et al.
Arzneimittel-Forschung, 62(5), 247-251 (2012-03-03)
The study aimed to compare and evaluate the bioequivalence of a new generic preparation of trospium chloride (CAS NO:10405-02-4) capsule (20 mg, test) and the available import tablet (20 mg , reference) for the requirement of state regulatory criteria in
V V Danilov et al.
Urologiia (Moscow, Russia : 1999), (4)(4), 15-20 (2010-10-26)
The analysis of 58 cases of overactive bladder has shown that detrusor activity is not linked with clinical symptoms but is caused by supra segmentary lesion of the nervous system. The clinical picture of overactive bladder fits the proposed neurophysiological
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门