所有图片(1)
About This Item
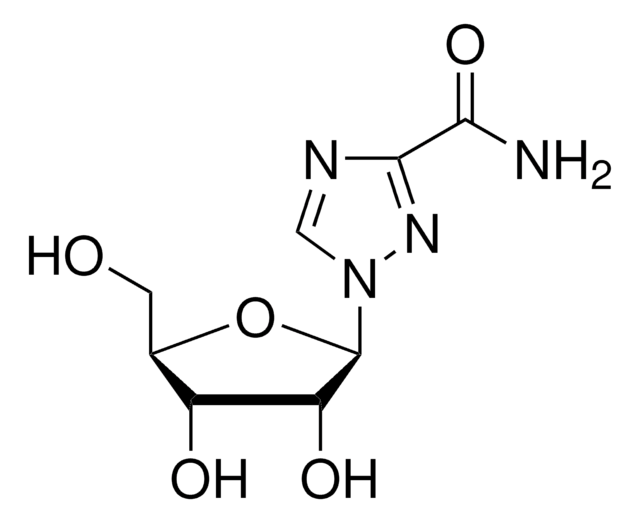
经验公式(希尔记法):
C8H12N4O5
CAS号:
分子量:
244.20
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推荐产品
等級
pharmaceutical primary standard
API 家族
ribavirin
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
SMILES 字串
NC(=O)c1ncn(n1)[C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O
InChI
1S/C8H12N4O5/c9-6(16)7-10-2-12(11-7)8-5(15)4(14)3(1-13)17-8/h2-5,8,13-15H,1H2,(H2,9,16)/t3-,4-,5-,8-/m1/s1
InChI 密鑰
IWUCXVSUMQZMFG-AFCXAGJDSA-N
基因資訊
human ... IMPDH1(3614)
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
嘌呤核苷酸生物合成中咪唑核苷酸中间体的类似物。
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Ribavirin EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
用于抵御多种人类病毒感染的抗病毒剂,特别是慢性肝炎C、HIV 和腺病毒。其代谢产物利巴韦林 5′-磷酸,是肌苷一磷酸 (IMP) 脱氢酶的抑制剂,但也有实验证据支持许多其他作用机制。
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
产品编号
说明
价格
訊號詞
Danger
危險聲明
危險分類
Muta. 2 - Repr. 1B
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Fred Poordad et al.
The New England journal of medicine, 370(21), 1973-1982 (2014-04-15)
Interferon-containing regimens for the treatment of hepatitis C virus (HCV) infection are associated with increased toxic effects in patients who also have cirrhosis. We evaluated the interferon-free combination of the protease inhibitor ABT-450 with ritonavir (ABT-450/r), the NS5A inhibitor ombitasvir
Eric Druyts et al.
Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 56(7), 961-967 (2012-12-18)
A systematic review and meta-analysis were conducted to examine the efficacy and safety of pegylated interferon (peg-IFN) alfa-2a and peg-IFN alfa-2b plus ribavirin (RBV) in children and adolescents with chronic hepatitis C virus (HCV). Medline, Embase, and Cochrane Central Register
Michael Manns et al.
Lancet (London, England), 384(9954), 1597-1605 (2014-08-01)
An unmet need exists for interferon-free and ribavirin-free treatments for chronic hepatitis C virus (HCV) infection. In this study, we assessed all-oral therapy with daclatasvir (NS5A replication complex inhibitor) plus asunaprevir (NS3 protease inhibitor) in patients with genotype 1b infection
Chen-Hua Liu et al.
Annals of internal medicine, 159(11), 729-738 (2013-12-04)
Data are limited on the efficacy and safety of pegylated interferon plus ribavirin for patients with hepatitis C virus genotype 1 (HCV-1) receiving hemodialysis. To compare the efficacy and safety of combination therapy with pegylated interferon plus low-dose ribavirin and
Tung-Hung Su et al.
Proceedings of the National Academy of Sciences of the United States of America, 110(19), 7844-7849 (2013-04-25)
MicroRNA-122 (miR-122) facilitates hepatitis C virus replication in vitro. Serum miR-122 has been implicated as a biomarker for various liver diseases; however, its role in chronic hepatitis C remains unclear. To address this issue, 126 patients with chronic hepatitis C
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门