PHR1658
茶苯海明
Pharmaceutical Secondary Standard; Certified Reference Material
别名:
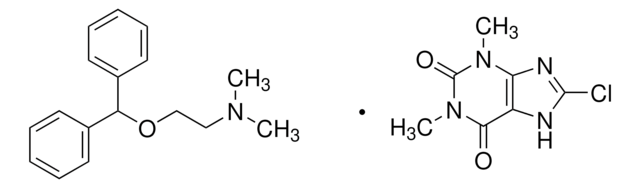
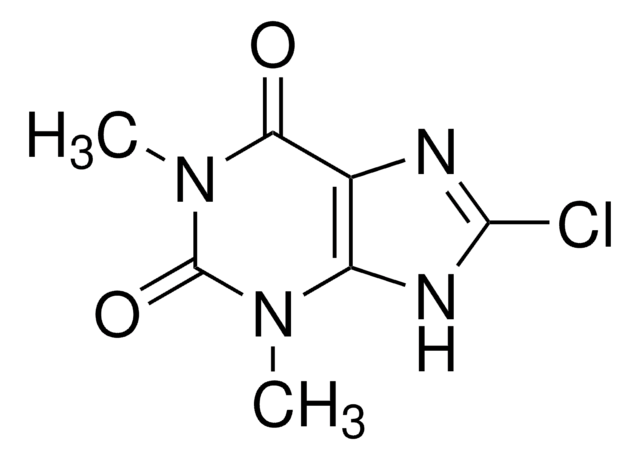
Dimenhydrinate, DMH, N-(2-Diphenylmethoxyethyl)-N,N-dimethylammonium 8-chlorotheophyllinate
登录查看公司和协议定价
所有图片(1)
About This Item
经验公式(希尔记法):
C17H21NO · C7H7ClN4O2
CAS号:
分子量:
469.96
EC號碼:
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推荐产品
等級
certified reference material
pharmaceutical secondary standard
品質等級
agency
traceable to Ph. Eur. D2000000
traceable to USP 1206006
API 家族
dimenhydrinate
CofA
current certificate can be downloaded
包裝
pkg of 1 g
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-30°C
SMILES 字串
CN1C(=O)N(C)c2nc(Cl)[nH]c2C1=O.CN(C)CCOC(c3ccccc3)c4ccccc4
InChI
1S/C17H21NO.C7H7ClN4O2/c1-18(2)13-14-19-17(15-9-5-3-6-10-15)16-11-7-4-8-12-16;1-11-4-3(9-6(8)10-4)5(13)12(2)7(11)14/h3-12,17H,13-14H2,1-2H3;1-2H3,(H,9,10)
InChI 密鑰
NFLLKCVHYJRNRH-UHFFFAOYSA-N
基因資訊
human ... HRH1(3269)
正在寻找类似产品? 访问 产品对比指南
一般說明
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Dimenhydrinate, a diphenhydramine salt of 8-chlorotheophylline, exhibits antihistaminic activity, with antimuscarinic and sedative effects. It finds its major application as an antiemetic drug in the prevention and treatment of motion sickness.
Dimenhydrinate, a diphenhydramine salt of 8-chlorotheophylline, exhibits antihistaminic activity, with antimuscarinic and sedative effects. It finds its major application as an antiemetic drug in the prevention and treatment of motion sickness.
應用
Dimenhydrinate may be used as a pharmaceutical reference standard for the determination of the analyte in traditional Chinese medicine and pharmaceutical formulations by various chromatography techniques.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
分析報告
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
其他說明
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
腳註
To see an example of a Certificate of Analysis for this material enter LRAC3017 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
推薦產品
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
相關產品
产品编号
说明
价格
其他客户在看
Optimization and validation of a method for the determination of caffeine, 8-chlorotheophylline and diphenhydramine by isocratic high-performance liquid chromatography: Stress test for stability evaluation.
Barbas C, et al.
Journal of Chromatography A, 870(1-2), 97-103 (2000)
Simultaneous determination of Dimenhydrinate, Cinnarizine and Cinnarizine impurity by TLC and HPLC chromatographic methods.
Ahmed AB, et al.
Bulletin of Faculty of Pharmacy, Cairo University , 55(1), 163-169 (2017)
HPLC and GC?MS screening of Chinese proprietary medicine for undeclared therapeutic substances.
Liu SY, et al.
Journal of Pharmaceutical and Biomedical Analysis, 24(5-6), 983-992 (2001)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门