推荐产品
等級
pharmaceutical primary standard
API 家族
pyrazinamide
製造商/商標名
EDQM
mp
189-191 °C (lit.)
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
SMILES 字串
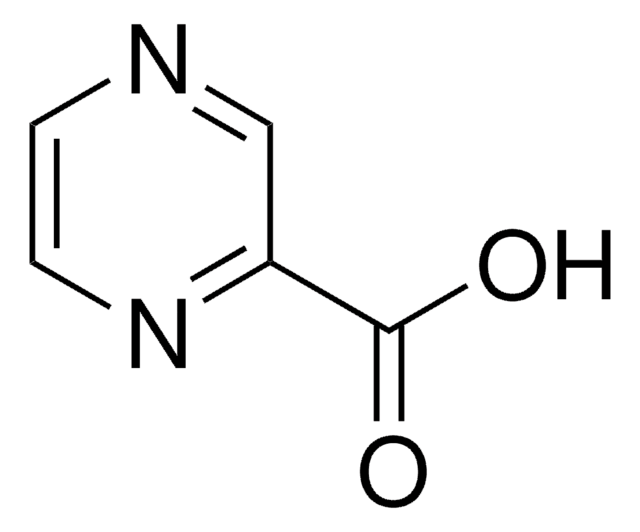
NC(=O)c1cnccn1
InChI
1S/C5H5N3O/c6-5(9)4-3-7-1-2-8-4/h1-3H,(H2,6,9)
InChI 密鑰
IPEHBUMCGVEMRF-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Pyrazinamide EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
A Somoskovi et al.
Respiratory research, 2(3), 164-168 (2001-11-01)
Multidrug-resistant (MDR) strains of Mycobacterium tuberculosis have emerged worldwide. In many countries and regions, these resistant strains constitute a serious threat to the efficacy of tuberculosis control programs. An important element in gaining control of this epidemic is developing an
L Gwaza et al.
Clinical pharmacology and therapeutics, 96(5), 580-588 (2014-07-06)
Approval of generic medicines is based on bioequivalence with the innovator product, but it is not unusual for generics to be interchanged with each other. This study investigated the differences in bioavailability between World Health Organization-prequalified antituberculosis generics by means
D A Mitchison et al.
Tuberculosis (Edinburgh, Scotland), 90(3), 177-181 (2010-04-13)
While we wait for improved new anti-tuberculosis drugs, the main aim for improving current treatment should be to optimize the use of the two current drugs, rifampicin and the pro-drug pyrazinamide, which are responsible to a similar extent for the
Tawanda Gumbo et al.
The Journal of antimicrobial chemotherapy, 69(9), 2420-2425 (2014-05-14)
To identify the pyrazinamide MIC above which standard combination therapy fails. MICs of pyrazinamide were determined for Mycobacterium tuberculosis isolates, cultured from 58 patients in a previous randomized clinical trial in Cape Town, South Africa. The MICs were determined using
X Gonzalo et al.
The Journal of antimicrobial chemotherapy, 69(11), 3001-3005 (2014-06-26)
Pyrazinamide is a key first-line tuberculosis drug. Reliable drug susceptibility testing (DST) data are of clinical importance, but in vitro testing is challenging since the activity of pyrazinamide is pH sensitive. The BACTEC MGIT 960 is considered the principal reference
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门