推荐产品
等級
pharmaceutical primary standard
API 家族
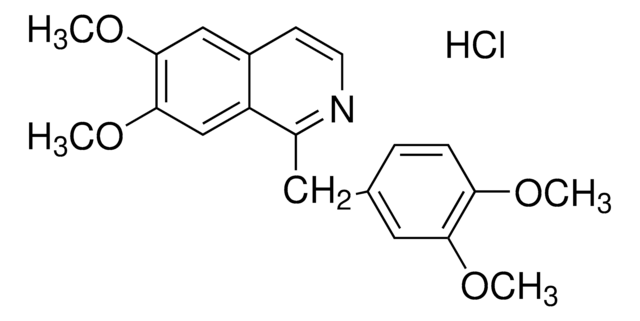
papaverine
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
COC1=C(OC)C=C(C(CC2=CC(OC)=C(OC)C=C2)=NC=C3)C3=C1.Cl
InChI
1S/C20H21NO4.ClH/c1-22-17-6-5-13(10-18(17)23-2)9-16-15-12-20(25-4)19(24-3)11-14(15)7-8-21-16;/h5-8,10-12H,9H2,1-4H3;1H
InChI 密鑰
UOTMYNBWXDUBNX-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Papaverine hydrochloride EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
Smooth muscle relaxant and cerebral vasodilator; phosphodiesterase inhibitor.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Bojidarka Ivanov et al.
Natural product communications, 7(5), 581-586 (2012-07-18)
The electronic structures, optical properties and molecular structures of a series of isoquinoline alkaloids resulting in the formation of papaverine, through a proposed biosynthetic pathway via S(+)-reticuline were elucidated. The mechanism of papaverine synthesis was studied by electronic absorption, diffuse
J M Mathis et al.
Neuroradiology, 39(2), 90-98 (1997-02-01)
Intra-arterial infusion of papaverine hydrochloride for subarachnoid hemorrhage-induced cerebral vasospasm has become an adjunctive endovascular therapy along with cerebral angioplasty. Our knowledge concerning the mechanism of action, method of administration and potential side effects of this therapeutic alternative are reviewed.
Isabel Desgagné-Penix et al.
The Plant journal : for cell and molecular biology, 72(2), 331-344 (2012-06-26)
Papaverine, a major benzylisoquinoline alkaloid in opium poppy (Papaver somniferum), is used as a vasodilator and antispasmodic. Conversion of the initial intermediate (S)-norcoclaurine to papaverine involves 3'-hydroxylation, four O-methylations and dehydrogenation. However, our understanding of papaverine biosynthesis remains controversial more
Y Yazir et al.
International journal of impotence research, 24(5), 185-190 (2012-05-11)
Epidemiological evidence showed that chronic ethanol consumption is a major risk factor in the development of impotence. The present study investigated the effects of carbachol-, electrical field stimulation (EFS)-, sodium nitroprusside (SNP)- and papaverine-induced relaxant responses in the isolated corpus
Doreen Schmidl et al.
Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie, 251(2), 515-520 (2012-12-04)
To investigate the effect of orally administered moxaverine (Kollateral forte®) on ocular blood flow in young healthy subjects. Sixteen healthy subjects (eight male/eight female) aged between 20 and 32 years were included in this placebo-controlled, double-masked, two-way crossover study. Volunteers
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门