推荐产品
等級
pharmaceutical primary standard
API 家族
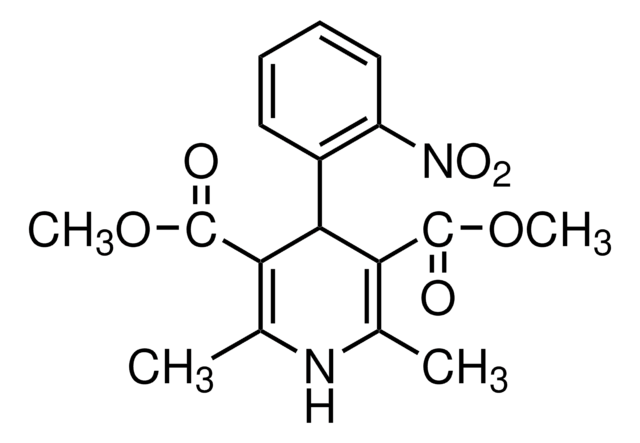
nifedipine
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
SMILES 字串
COC(=O)c1c(C)nc(C)c(C(=O)OC)c1-c2ccccc2[N+]([O-])=O
InChI
1S/C17H16N2O6/c1-9-13(16(20)24-3)15(14(10(2)18-9)17(21)25-4)11-7-5-6-8-12(11)19(22)23/h5-8H,1-4H3
InChI 密鑰
UMQHJQGNGLQJPF-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Nifedipine impurity A EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
CYP3A4 nifedipine metabolite. Nifedipine (parent compound) is an antianginal and antihypertensive agent.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
产品编号
说明
价格
訊號詞
Danger
危險聲明
危險分類
Eye Dam. 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Occurrence and measurement of nifedipine and its nitropyridine derivatives in human blood plasma.
J Dokladalova et al.
Journal of chromatography, 231(2), 451-458 (1982-09-10)
D G Waller et al.
British journal of clinical pharmacology, 18(6), 951-954 (1984-12-01)
Oral administration of nifedipine (20 and 30 mg tablets) to six volunteers was associated with a bioavailability of 0.43 and the presence of its nitropyridine analogue in the plasma. This metabolite was present in only trace amounts in samples taken
J L Born et al.
Chemical research in toxicology, 2(1), 57-59 (1989-01-01)
The primary deuterium isotope effect on Vm for the microsomal oxidation of the dihydropyridine calcium entry blocker nifedipine [4-(2-nitrophenyl)-2,6-dimethyl-3,5- bis(methoxycarbonyl)-1,4-dihydropyridine] has been measured. The magnitude of the kinetic isotope effect, 6.7, suggests that the rate-limiting step in the mechanism of
Tsai-Shin Chiang et al.
PloS one, 9(4), e94885-e94885 (2014-04-16)
Human hepatoma cell lines are commonly used as alternatives to primary hepatocytes for the study of drug metabolism in vitro. However, the phase I cytochrome P450 (CYP) enzyme activities in these cell lines occur at a much lower level than
Camille C Savary et al.
Drug metabolism and disposition: the biological fate of chemicals, 42(8), 1235-1240 (2014-05-17)
Humans are usually exposed to several pesticides simultaneously; consequently, combined actions between pesticides themselves or between pesticides and other chemicals need to be addressed in the risk assessment. Many pesticides are efficient activators of pregnane X receptor (PXR) and/or constitutive
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门