推荐产品
等級
pharmaceutical primary standard
API 家族
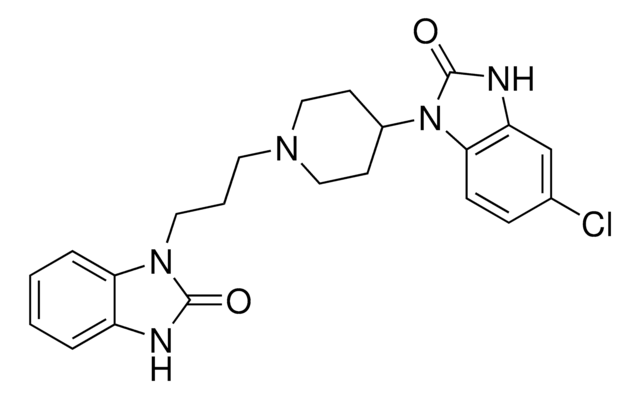
droperidol
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
SMILES 字串
Fc1ccc(cc1)C(=O)CCCN2CCC(=CC2)N3C(=O)Nc4ccccc34
InChI
1S/C22H22FN3O2/c23-17-9-7-16(8-10-17)21(27)6-3-13-25-14-11-18(12-15-25)26-20-5-2-1-4-19(20)24-22(26)28/h1-2,4-5,7-11H,3,6,12-15H2,(H,24,28)
InChI 密鑰
RMEDXOLNCUSCGS-UHFFFAOYSA-N
基因資訊
human ... DRD2(1813) , DRD3(1814) , DRD4(1815) , HTR2A(3356)
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Droperidol EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Gregory A Nuttall et al.
Anesthesiology, 118(2), 382-386 (2013-01-08)
The Food and Drug Administration issued a black box warning regarding the use of droperidol and the potential for torsade de pointes. The primary objective of this retrospective study was to determine if low-dose (0.625 mg) droperidol administration was associated
Christopher Spevak et al.
Pain medicine (Malden, Mass.), 13(8), 1072-1080 (2012-06-12)
The most feared drug-induced complication is fatal cardiac arrest. Torsades de pointes (TdP) is a polymorphic ventricular tachycardia occurring in the setting of a QT interval prolongation and is the most frequent type of drug-induced pro-arrhythmia. The most common mechanism
Esther W Chan et al.
Annals of emergency medicine, 61(1), 72-81 (2012-09-18)
Parenteral benzodiazepines or antipsychotics are often used to manage acute agitation in emergency department (ED) settings in which alternative strategies have failed or are not feasible. There are scant data comparing parenteral medication regimens. We aim to determine the efficacy
To add or not to add? An empirical study on droperidol and intravenous patient-controlled analgesia.
Yi-Min Kuo et al.
Journal of the Chinese Medical Association : JCMA, 75(5), 227-233 (2012-05-29)
Droperidol is commonly added to intravenous patient-controlled analgesia (IVPCA) regimens as an antiemetic agent. Although some studies have demonstrated its safety and efficacy, it is not clear whether adding droperidol to IVPCA infusate without an extra loading dose can effectively
G Ormel et al.
Acta anaesthesiologica Scandinavica, 55(10), 1196-1205 (2011-11-19)
Prophylactic dexamethasone, ondansetron and droperidol have a documented effect on post-operative nausea and vomiting (PONV). Still, there is a lack of studies investigating the effect of adding dexamethasone to ondansetron and droperidol in order to treat established PONV. In this
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门

![1-[2-(1H-1,2,4-Triazol-1-yl)ethyl]piperazine trihydrochloride hydrate AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/368/613/f6871331-830e-4854-994c-7f159b99e330/640/f6871331-830e-4854-994c-7f159b99e330.png)