推荐产品
製造商/商標名
BP
drug control
estupefaciente (Spain); Decreto Lei 15/93: Tabela IA (Portugal)
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.
For further information and support please go to the website of the issuing Pharmacopoeia.
For further information and support please go to the website of the issuing Pharmacopoeia.
應用
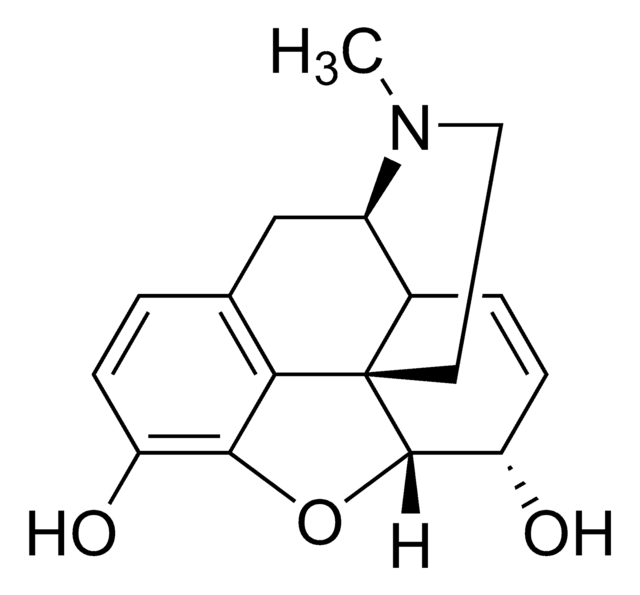
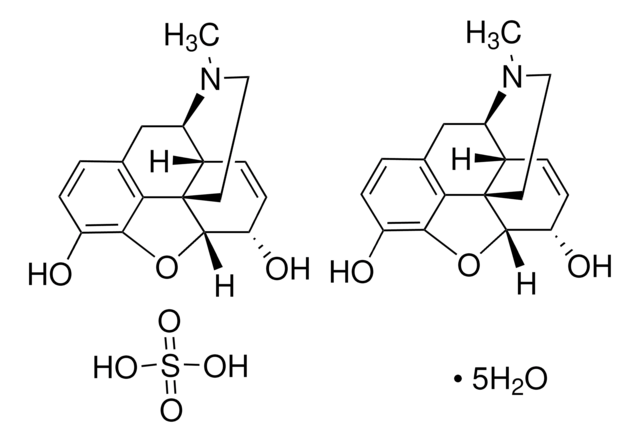
Codeine phosphate BP Reference standard, intended for use in laboratory tests only as specifically prescribed in the British Pharmacopoeia.
Also used in monographs such as:Paracetamol, Codeine Phosphate and Caffeine Capsules Co-dydramol Tablets Co-codamol Capsules Co-codamol Tablets Co-codamol Effervescent Tablets Paracetamol, Codeine Phosphate and Caffeine Tablets Morphine Tablets Morphine Prolonged-release Tablets Dihydrocodeine Prolonged-release Tablets Morphine Prolonged-release Capsules Morphine Granules for Oral Suspension Dihydrocodeine Oral Solution Co-codaprin Tablets Dihydrocodeine Injection Morphine Oral Solution Dihydrocodeine Tablets Morphine Suppositories Morphine Capsules Morphine Sulfate Injection Codeine Phosphate Oral Solution
Also used in monographs such as:
包裝
Unit quantity: 200 mg. Subject to change. The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity please visit British Pharmacopoeia
其他說明
Sales restrictions may apply.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门