推荐产品
等級
analytical standard
化驗
≥98.0% (HPLC)
儲存期限
limited shelf life, expiry date on the label
應用
clinical testing
格式
neat
儲存溫度
2-8°C
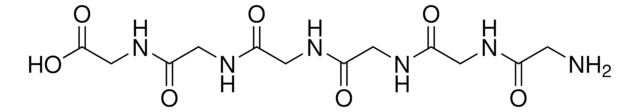
InChI
1S/C11H11NO3/c13-10(12-8-11(14)15)7-6-9-4-2-1-3-5-9/h1-7H,8H2,(H,12,13)(H,14,15)/b7-6+
InChI 密鑰
YAADMLWHGMUGQL-VOTSOKGWSA-N
生化/生理作用
Cinnamoylglycine is known as a urinary metabolite in man, although whether it is formed de novo from plant cinnamate or is a plant product excreted unchanged has not been conclusively demonstrated. When cinnamoylglycine occurs naturally it is probably a food constituent excreted unchanged. It is not found when small quantities (0.5-6 g) of cinnamic acid are fed to man, but by analogy with animal experiments may be produced when much larger quantities are given.
推薦產品
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
G K Brown et al.
Journal of chromatography, 145(2), 177-184 (1978-03-01)
The profile of high boiling point organic acids in urine samples from both normal subjects and patients suspected of having some form of metabolic disorder has been determined by combined gas chromatography-mass spectrometry. Fifteen different compounds eluting after hippuric acid
J A Hoskins et al.
Biomedical mass spectrometry, 11(6), 296-300 (1984-06-01)
The enzyme phenylalanine ammonia lyase taken orally has been found to reduce the rise in blood phenylalanine that normally occurs following a protein meal. Therefore the enzyme has a potential use in the management of the genetic disease phenylketonuria. The
Bejan J Saeedi et al.
Cell metabolism, 31(5), 956-968 (2020-03-28)
Many studies have suggested a role for gut-resident microbes (the "gut microbiome") in modulating host health; however, the mechanisms by which they impact systemic physiology remain largely unknown. In this study, metabolomic and transcriptional profiling of germ-free and conventionalized mouse
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门