所有图片(1)
About This Item
经验公式(希尔记法):
C20H26O6
分子量:
362.42
Beilstein:
6611290
EC號碼:
MDL號碼:
分類程式碼代碼:
41116105
PubChem物質ID:
NACRES:
NA.21
推荐产品
化驗
≥95.0% (HPLC)
形狀
solid
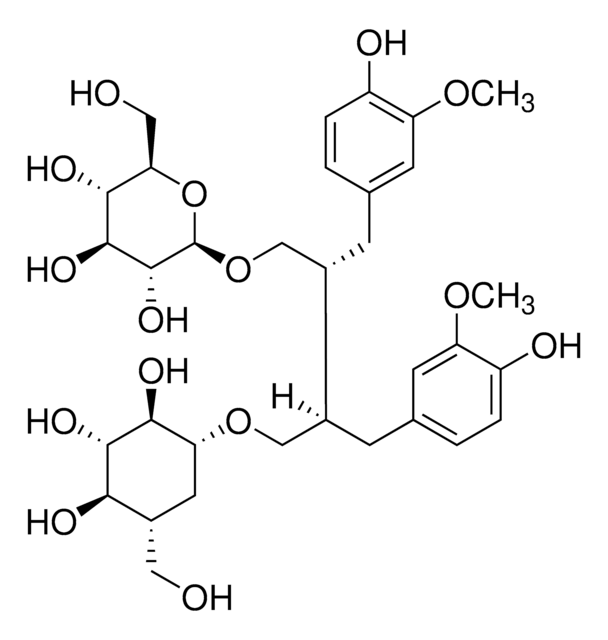
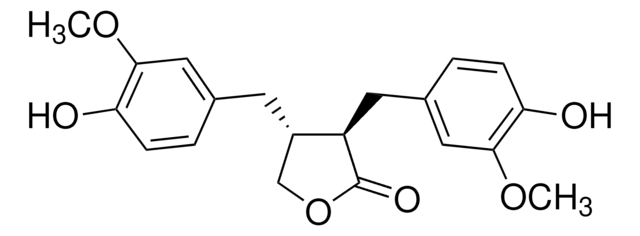
SMILES 字串
OC[C@@H]([C@H](CO)CC1=CC=C(O)C(OC)=C1)CC2=CC(OC)=C(O)C=C2
InChI
1S/C20H26O6/c1-25-19-9-13(3-5-17(19)23)7-15(11-21)16(12-22)8-14-4-6-18(24)20(10-14)26-2/h3-6,9-10,15-16,21-24H,7-8,11-12H2,1-2H3/t15-,16-/m0/s1
InChI 密鑰
PUETUDUXMCLALY-HOTGVXAUSA-N
應用
异豆香脂树脂是异豆脂树脂的一种代谢产物,是亚麻籽中的一种抗氧化剂。
包裝
无底玻璃瓶。内含物在插入的融合锥体内。
外觀
(R*,R*)-非对映异构体的对映体混合物
其他說明
功能性食品的成分。具有抗氧化剂活性的亚麻木酚素
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Carol J Fabian et al.
Cancer prevention research (Philadelphia, Pa.), 3(10), 1342-1350 (2010-08-21)
Preclinical and correlative studies suggest reduced breast cancer with higher lignan intake or blood levels. We conducted a pilot study of modulation of risk biomarkers for breast cancer in premenopausal women after administration of the plant lignan secoisolariciresinol given as
Shiori Tominaga et al.
Food & function, 3(1), 76-82 (2011-10-28)
Flaxseed lignan, secoisolariciresinol has been reported to possess health benefits. We previously synthesized each stereoisomer of secoisolariciresinol and found that (-)-secoisolariciresinol reduces lipid accumulation and induces adiponectin production in 3T3-L1 adipocytes. Here we show the effects of (-)-secoisolariciresinol on high-fat
Cheng-Zhi Wang et al.
BMC microbiology, 10, 115-115 (2010-04-20)
The effects of enterolignans, e.g., enterodiol (END) and particularly its oxidation product, enterolactone (ENL), on prevention of hormone-dependent diseases, such as osteoporosis, cardiovascular diseases, hyperlipemia, breast cancer, colon cancer, prostate cancer and menopausal syndrome, have attracted much attention. To date
D D Kitts et al.
Molecular and cellular biochemistry, 202(1-2), 91-100 (2000-03-08)
The antioxidant activities of the flaxseed lignan secoisolariciresinol diglycoside (SDG) and its mammalian lignan metabolites, enterodiol (ED) and enterolactone (EL), were evaluated in both lipid and aqueous in vitro model systems. All three lignans significantly (p < or = 0.05)
Hisashi Nishiwaki et al.
Bioscience, biotechnology, and biochemistry, 75(9), 1735-1739 (2011-09-08)
The larvicidal activity against Culex pipiens of all stereoisomers of dihydroguaiaretic acid (DGA) and secoisolariciresinol was measured, and these DGAs were found to be potent. Sixteen (-)-DGA derivatives were then newly synthesized to analyze their structure-activity relationship. Two derivatives monohydroxylated
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门