推荐产品
等級
analytical standard
儲存期限
limited shelf life, expiry date on the label
技術
HPLC: suitable
gas chromatography (GC): suitable
折射率
n20/D 1.582 (lit.)
bp
89 °C/16 mmHg (lit.)
mp
−8 °C (lit.)
密度
0.997 g/mL at 25 °C (lit.)
應用
environmental
形式
neat
SMILES 字串
C1Cc2ccccc2C=C1
InChI
1S/C10H10/c1-2-6-10-8-4-3-7-9(10)5-1/h1-3,5-7H,4,8H2
InChI 密鑰
KEIFWROAQVVDBN-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
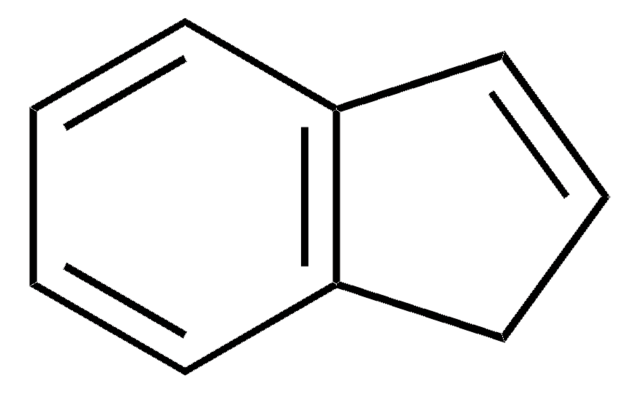
1,2-Dihydronaphthalene is a bicyclic hydrocarbon, which resembles naphthalene but shows partial unsaturation in one of its rings. Its derivatives find wide applications in natural compounds of therapeutic interest.
應用
1,2-Dihydronaphthalene may be used as an analytical standard for the determination of the analyte in polycyclic aromatic hydrocarbon (PAH) mixtures by gas chromatography technique.
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
152.6 °F - closed cup
閃點(°C)
67 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
The synthesis of novel dihydronaphthalenes and benzofluorenes
Novel Selenium-Mediated Rearrangements and Cyclisations, 77(6), 1210-1212 (2012)
Retention indices for programmed-temperature capillary-column gas chromatography of polycyclic aromatic hydrocarbons
Lee ML, et al.
Analytical Chemistry, 51(6), 768-773 (1979)
Prediction of gas chromatographic retention indexes of polycyclic aromation compounds and nitrated polycyclic aromatic compounds
Rohrbaugh RH and Jurs PC
Analytical Chemistry, 58(6), 1210-1212 (1986)
S L Eaton et al.
Applied and environmental microbiology, 62(12), 4388-4394 (1996-12-01)
The substrate oxidation profiles of Sphingomonas yanoikuyae B1 biphenyl-2,3-dioxygenase and cis-biphenyl dihydrodiol dehydrogenase activities were examined with 1,2-dihydronaphthalene and various cis-diols as substrates. m-Xylene-induced cells of strain B1 oxidized 1,2-dihydronaphthalene to (-)-(1R,2S)-cis-1,2-dihydroxy-1,2-3,4-tetrahydronaphthalene as the major product (73% relative yield). Small
Keith Smith et al.
Chemical communications (Cambridge, England), (8)(8), 886-887 (2002-07-19)
We have successfully prepared an unsymmetrical analogue of a Katsuki-type salen ligand having a single hydroxyalkyl group at its 6-position, and also its Mn(III) complex; attachment of the complex to a polymer gives a highly enantioselective and recoverable catalyst for
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门