所有图片(1)
About This Item
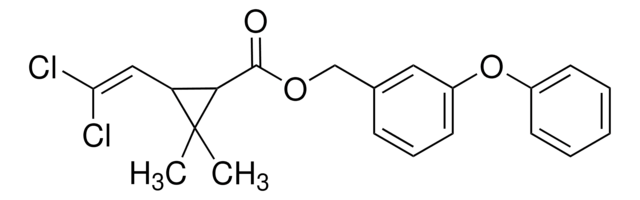
经验公式(希尔记法):
C16H14Cl2O4
CAS号:
分子量:
341.19
Beilstein:
2224754
EC號碼:
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推荐产品
等級
analytical standard
品質等級
產品線
PESTANAL®
儲存期限
limited shelf life, expiry date on the label
技術
HPLC: suitable
gas chromatography (GC): suitable
mp
42-48 °C (if solid)
應用
agriculture
environmental
格式
neat
SMILES 字串
COC(=O)C(C)Oc1ccc(Oc2ccc(Cl)cc2Cl)cc1
InChI
1S/C16H14Cl2O4/c1-10(16(19)20-2)21-12-4-6-13(7-5-12)22-15-8-3-11(17)9-14(15)18/h3-10H,1-2H3
InChI 密鑰
BACHBFVBHLGWSL-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Diclofop-methyl is an early post-emergence phenoxypropanoic acid herbicide. Its mode of action involves inhibition of acetyl CoA carboxylase (ACCase) activity, which in turn decreases fatty acid synthesis in weeds and inhibits photosynthesis and the activity of meristem.
應用
Diclofop-methyl may be used as a reference standard for the determination of diclofop-methyl in soil samples by gas-liquid chromatography (GLC).
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
法律資訊
PESTANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
未找到合适的产品?
试试我们的产品选型工具.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Skin Sens. 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Jose L De Prado et al.
Journal of agricultural and food chemistry, 53(6), 2185-2191 (2005-03-17)
Three diclofop-methyl (DM) resistant biotypes of Lolium rigidum (R1, R2, and R3) were found in different winter wheat fields in Spain, continuously treated with DM, DM + chlortoluron, or DM + isoproturon. Herbicide rates that inhibited shoot growth by 50%
Vanesa Guillén-Casla et al.
Journal of agricultural and food chemistry, 56(7), 2303-2309 (2008-03-15)
Simple one- and two-dimensional high-performance liquid chromatography (HPLC) methods for the simultaneous enantiomeric determination of alkyloxyphenoxypropionic acid herbicides is presented. Compounds studied were ( R, S)-2-[4-(2,4-dichlorophenoxy)phenoxy]propionic acid (diclofop-acid) and ( R, S)-2-[4-(2,4-dichlorophenoxy)]methyl propionate (diclofop-methyl). Mobile phases necessary to separate their
Xiyun Cai et al.
Environmental toxicology and chemistry, 26(5), 970-975 (2007-05-25)
Information on the effects of pesticide degradation on aquatic organisms is needed to properly evaluate the ecotoxicity arising from the use of pesticides and for aquatic risk assessment. This work evaluated the toxicity of diclofop-methyl (DM) and its two major
Fatma Ünal et al.
Drug and chemical toxicology, 34(4), 390-395 (2011-07-01)
Diclofop-methyl (DM) is a chlorophenoxy derivative used in large quantities for the control of annual grasses in grain and vegetable crops. In this study, the genotoxic effects of DM were investigated by measuring chromosomal aberrations (CAs) in mouse bone-marrow cells
Qin Yu et al.
Planta, 230(4), 713-723 (2009-07-16)
This study investigates mechanisms of multiple resistance to glyphosate, acetyl-coenzyme A carboxylase (ACCase) and acetolactate synthase (ALS)-inhibiting herbicides in two Lolium rigidum populations from Australia. When treated with glyphosate, susceptible (S) plants accumulated 4- to 6-fold more shikimic acid than
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门