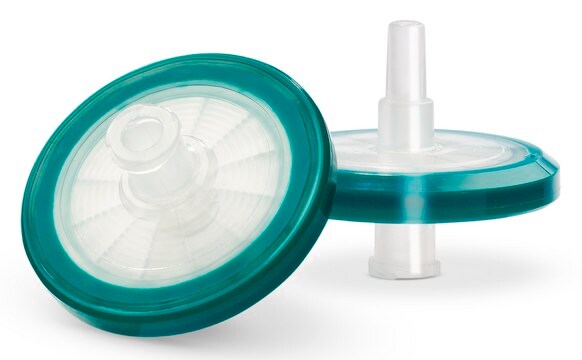
推荐产品
材料
mixed cellulose esters (MCE) membrane
modified acrylic housing
品質等級
無菌
sterile; ethylene oxide treated
產品線
Millex®
特點
holdup volume< 0.1 mL
hydrophilic
FDA registered
medical device
製造商/商標名
Millex®
參數
10 bar max. inlet pressure (145 psi)
45 °C max. temp.
直徑
33 mm
過濾面積
4.52 cm2
inlet to outlet H
27 mm
容積
100 mL
基質
MF-Millipore™
孔徑
0.8 μm pore size
接頭
female Luer-Lok® inlet
male Luer outlet slip
運輸包裝
ambient
一般說明
Millex®-AA 过滤器(无菌)是一种医疗设备,可用作针头过滤器,用于患者直接护理和药房加药配液应用中小体积溶液的澄清。
應用
- 临床获益:去除 临床用溶液中大于 滤膜额定孔径的微生物、颗粒、沉淀物和未溶解粉末。
- 医院药房: 在加药配液时,对小体积的蛋白药物、诊断 成像剂、化疗药物、水溶液或水 进行澄清处理。
- 患者直接护理: 去除硬膜外麻醉剂、其他液体麻醉剂以及眼科、耳科和 其他外科手术中使用的冲洗液中的 微粒。
特點和優勢
- 滤膜 的双向支持
- 去除大于滤膜额定孔径的 微生物、颗粒、沉淀物和未溶解粉末
- 一次性、无热原、无毒
- 封装在改性 丙烯酸共聚物 (MMA) 外壳中的 混合纤维素酯 (MCE) 薄膜过滤器
- 与大多数水溶液兼容
- 经 CE 认证,并在 FDA 注册
其他說明
设备仅可用于医疗和/或实验室专业用途。
法律資訊
Luer-Lok is a registered trademark of Becton-Dickinson & Co.
MF-Millipore is a trademark of Merck KGaA, Darmstadt, Germany
Millex is a registered trademark of Merck KGaA, Darmstadt, Germany
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门