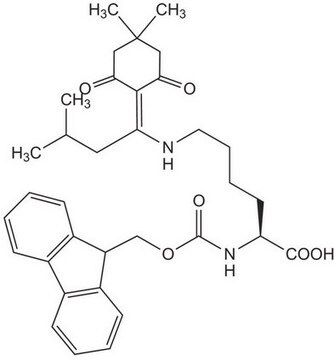
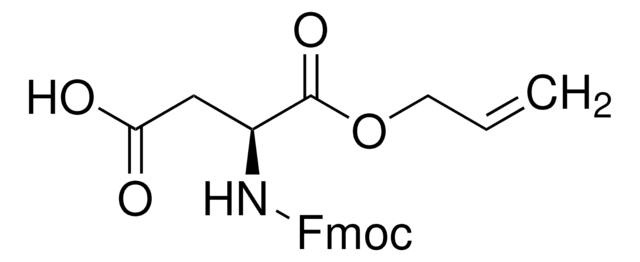
推荐产品
品質等級
產品線
Novabiochem®
化驗
≥95.0% (HPLC)
≥97% (TLC)
形狀
powder
反應適用性
reaction type: Fmoc solid-phase peptide synthesis
製造商/商標名
Novabiochem®
應用
peptide synthesis
官能基
carboxylic acid
儲存溫度
15-25°C
一般說明
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references
[1] W. C. Chan, et al. (1995) J. Chem. Soc., Chem. Commun., 2209.
[2] S. Künzel, et al. Poster 17 presented at Solid Phase Synthesis & Combinatorial Libraries, Southampton, September 2001.
[3] K. F. Medzihradszky, et al. (2002) Lett. Pept. Sci., 8, 1.
[4] Albericio, et al.Poster 44 presented at American Peptide Symposium, San Diego 2005..
[5] M. Cudic, et al. in ′Peptides 2000, Proc. 26th European Peptide Symposium′, J. Martinez & J.-A. Fehrentz (Eds), Paris, Editions EDK, 2001, pp. 203.
[6] M. Cudic, et al. (2000) Tetrahedron Lett., 41, 4527.
[7] J. P. Malkinson, et al. (2003) Org. Lett., 5, 5051.
聯結
分析報告
Appearance of substance (visual): powder
Identity (IR): passes test
Enantiomeric purity: ≥ 99.0 % (a/a)
Purity (TLC(157A)): ≥ 97 %
Purity (TLC(CMA2)): ≥ 97 %
Assay (HPLC, area%): ≥ 95.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
Water (K. F.): ≤ 1.0 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
法律資訊
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
商品
Novabiochem® product range has one of the largest collections of orthogonally and quasi-orthogonally protected tri-functional amino acids. These derivatives are useful tools for the synthesis of cyclic and branched peptides and peptides carrying side-chain modifications.
Novabiochem® product range has one of the largest collections of orthogonally and quasi-orthogonally protected tri-functional amino acids. These derivatives are useful tools for the synthesis of cyclic and branched peptides and peptides carrying side-chain modifications.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门