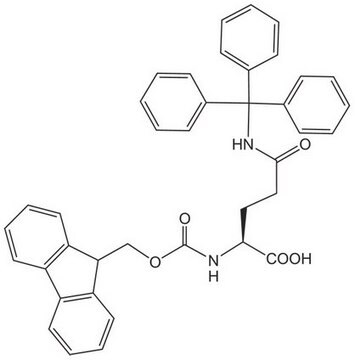
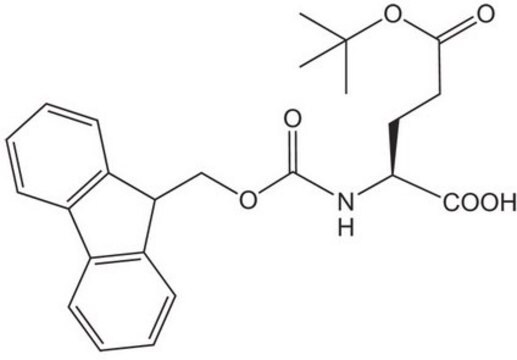
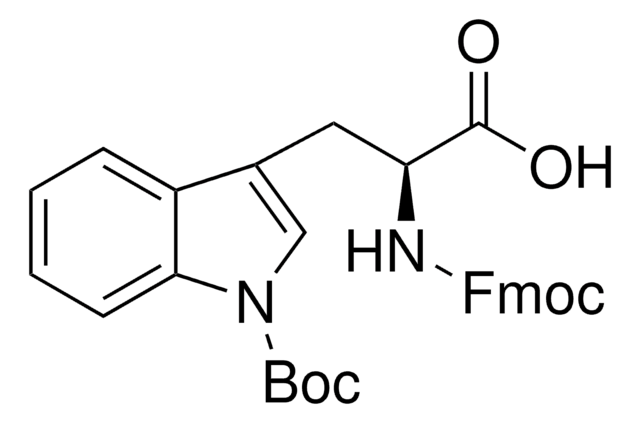
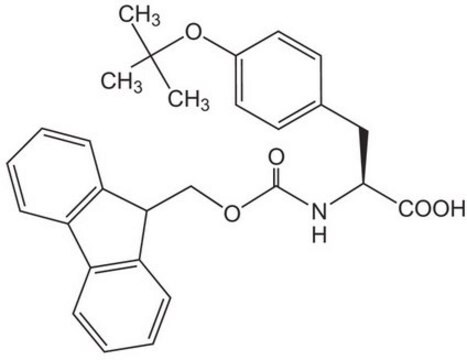
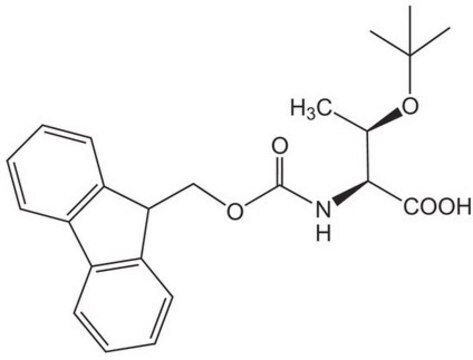
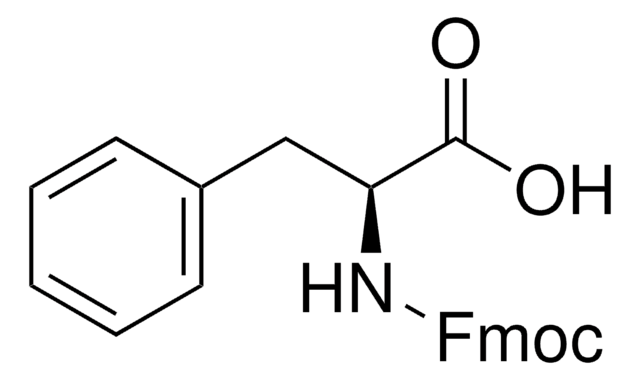
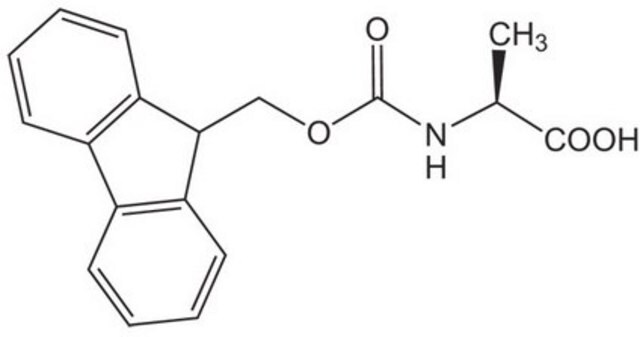
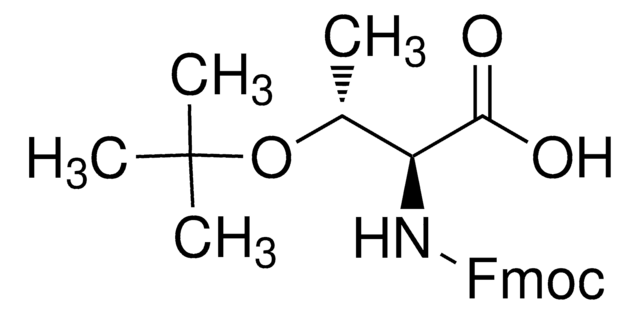
推荐产品
品質等級
產品線
Novabiochem®
化驗
≥90.0% (acidimetric)
≥97.5% (HPLC)
≥98% (TLC)
形狀
powder
反應適用性
reaction type: Fmoc solid-phase peptide synthesis
製造商/商標名
Novabiochem®
應用
peptide synthesis
官能基
Boc
Fmoc
儲存溫度
15-25°C
InChI
1S/C31H30N2O6/c1-31(2,3)39-30(37)33-17-19(20-10-8-9-15-27(20)33)16-26(28(34)35)32-29(36)38-18-25-23-13-6-4-11-21(23)22-12-5-7-14-24(22)25/h4-15,17,25-26H,16,18H2,1-3H3,(H,32,36)(H,34,35)/t26-/m0/s1
InChI 密鑰
ADOHASQZJSJZBT-SANMLTNESA-N
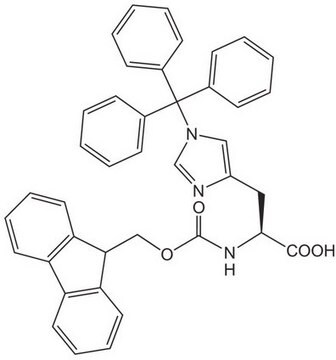
一般說明
High purity Fmoc-protected amino acid for research and process production of peptides, with very low levels of dipeptide, free-amino acids and acetic acid impurities.
The use of this N-in-Boc protected derivative overcomes most of the problems associated with the preparation of Trp containing-peptides by Fmoc SPPS [1]. Cleavage with TFA generates an N-in-carboxy indole which protects the Trp from alkylation [1,2,3] and sulfonation [1,4,5,6,7]. The N-in-carboxy group is removed under aqueous conditions during normal work-up of the peptide.
Associated Protocols and Technical Articles
Fmoc-amino acids for Peptide Production
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references
[1] P. White in ′Peptides, Chemistry & Biology, Proc. 12th American Peptide Symposium′, J. A. Smith & J. E. Rivier (Eds), ESCOM, Leiden, 1992, pp. 537.
[2] B. Riniker, et al. (1993) Tetrahedron, 49, 9307.
[3] T. Johnson, et al. (1993) J. Chem. Soc., Chem. Commun., 369.
[4] H. Choi, et al. (1993) Int. J. Peptide Protein Res., 42, 58.
[5] C. G. Fields, et al. (1993) Tetrahedron Lett., 34, 6661.
[6] T. Lescrinier, et al. (1995) Lett. Pept. Sci., 2, 225.
[7] M. Noda & M. Kiffe (1997) J. Peptide Res., 50, 329.
The use of this N-in-Boc protected derivative overcomes most of the problems associated with the preparation of Trp containing-peptides by Fmoc SPPS [1]. Cleavage with TFA generates an N-in-carboxy indole which protects the Trp from alkylation [1,2,3] and sulfonation [1,4,5,6,7]. The N-in-carboxy group is removed under aqueous conditions during normal work-up of the peptide.
Associated Protocols and Technical Articles
Fmoc-amino acids for Peptide Production
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references
[1] P. White in ′Peptides, Chemistry & Biology, Proc. 12th American Peptide Symposium′, J. A. Smith & J. E. Rivier (Eds), ESCOM, Leiden, 1992, pp. 537.
[2] B. Riniker, et al. (1993) Tetrahedron, 49, 9307.
[3] T. Johnson, et al. (1993) J. Chem. Soc., Chem. Commun., 369.
[4] H. Choi, et al. (1993) Int. J. Peptide Protein Res., 42, 58.
[5] C. G. Fields, et al. (1993) Tetrahedron Lett., 34, 6661.
[6] T. Lescrinier, et al. (1995) Lett. Pept. Sci., 2, 225.
[7] M. Noda & M. Kiffe (1997) J. Peptide Res., 50, 329.
應用
- Highly active antibacterial ferrocenoylated or ruthenocenoylated Arg-Trp peptides can be discovered by an L-to-D substitution scan: Utilizes Fmoc-Trp(Boc)-OH in the synthesis of peptides designed to enhance antibacterial activity, demonstrating its role in the development of new antimicrobial agents (Albada et al., 2014).
- Nanoparticles generated from a tryptophan derivative: physical characterization and anti-cancer drug delivery: Explores the use of Fmoc-Trp(Boc)-OH in nanoparticle formation for targeted drug delivery systems, particularly in cancer therapy (Dube et al., 2017).
聯結
Replaces: 04-12-1103
分析報告
Colour (visual): white to off white
Appearance of substance (visual): powder
Colour index (0,5 M in DMF): ≤ 150 Hazen
Identity (IR): passes test
Enantiomeric purity: ≥ 99.7 % (a/a)
Purity (HPLC): ≥ 97.5 % (a/a)
Fmoc-ß-Ala-OH (HPLC): ≤ 0.3 % (a/a)
Fmoc-ß-Ala-Trp (Boc)-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-Trp(Boc)-Trp(Boc)-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-Trp-OH (HPLC): ≤ 1.0 % (a/a)
Assay free amino acid (HPLC): ≤ 0.2 %
Solubility (25 mmole in 50 ml DMF): clear soluble
Purity (TLC(011A)): ≥ 98 %
Purity (TLC(0811)): ≥ 98 %
Assay (acidimetric): ≥ 90.0 %
Water (K. F.): ≤ 2.0 %
Ethyl acetate (HS-GC): ≤ 2.5 %
Acetate (IC): ≤ 0.10 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
Appearance of substance (visual): powder
Colour index (0,5 M in DMF): ≤ 150 Hazen
Identity (IR): passes test
Enantiomeric purity: ≥ 99.7 % (a/a)
Purity (HPLC): ≥ 97.5 % (a/a)
Fmoc-ß-Ala-OH (HPLC): ≤ 0.3 % (a/a)
Fmoc-ß-Ala-Trp (Boc)-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-Trp(Boc)-Trp(Boc)-OH (HPLC): ≤ 0.1 % (a/a)
Fmoc-Trp-OH (HPLC): ≤ 1.0 % (a/a)
Assay free amino acid (HPLC): ≤ 0.2 %
Solubility (25 mmole in 50 ml DMF): clear soluble
Purity (TLC(011A)): ≥ 98 %
Purity (TLC(0811)): ≥ 98 %
Assay (acidimetric): ≥ 90.0 %
Water (K. F.): ≤ 2.0 %
Ethyl acetate (HS-GC): ≤ 2.5 %
Acetate (IC): ≤ 0.10 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
法律資訊
Novabiochem is a registered trademark of Merck KGaA, Darmstadt, Germany
未找到合适的产品?
试试我们的产品选型工具.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门