324890
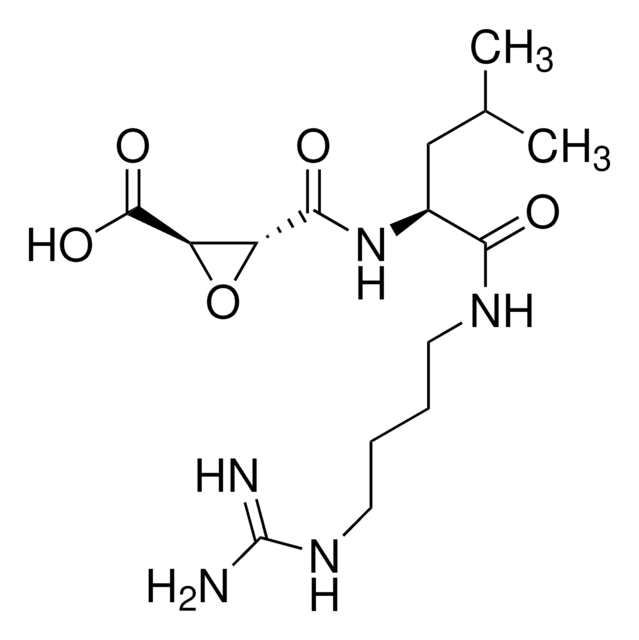
E-64 Protease Inhibitor
The E-64 Protease Inhibitor, also referenced under CAS 66701-25-5, controls the biological activity of E-64 Protease. This small molecule/inhibitor is primarily used for Protease Inhibitors applications.
别名:
E-64 Protease Inhibitor, (2 R,3 R)-3-(( S)-1-(4-Guanidinobutylamino)-4-methyl-1-oxopentan-2-ylcarbamoyl)oxirane-2-carboxylic acid, trans-Epoxysuccinyl-L-leucylamido(4-guanidino)butane, L- trans-3-Carboxyoxira, (2R,3R)-3-((S)-1-(4-Guanidinobutylamino)-4-methyl-1-oxopentan-2-ylcarbamoyl)oxirane-2-carboxylic acid, trans-Epoxysuccinyl-L-leucylamido(4-guanidino)butane, L-trans-3-Carboxyoxiran-R
About This Item
推荐产品
品質等級
化驗
≥98% (HPLC)
形狀
solid
製造商/商標名
Calbiochem®
儲存條件
OK to freeze
顏色
white
溶解度
water: 20 mg/mL
DMSO: 25 mg/mL
運輸包裝
ambient
儲存溫度
−20°C
SMILES 字串
N([C@@H](CC(C)C)C(=O)NCCCCN\C(=N/[H])\N)C(=O)[C@@H]1O[C@H]1C(=O)O
InChI
1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10+,11+/m0/s1
InChI 密鑰
LTLYEAJONXGNFG-HBNTYKKESA-N
一般說明
生化/生理作用
cysteine proteases
警告
重構
其他說明
Sarin, A., et al. 1994. J. Immunol.153, 862.
Sarin, A., et al. 1993. J. Exp. Med. 178, 1693.
Barrett, A.J. 1982. Biochem. J.201, 189.
法律資訊
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门