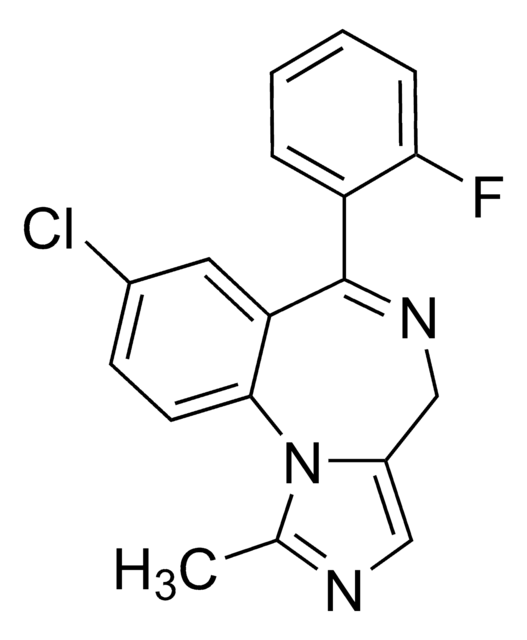
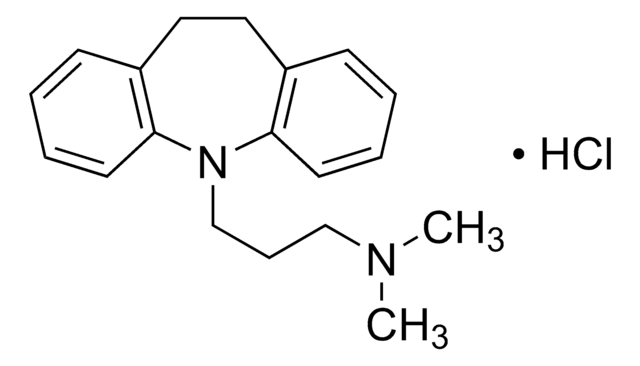
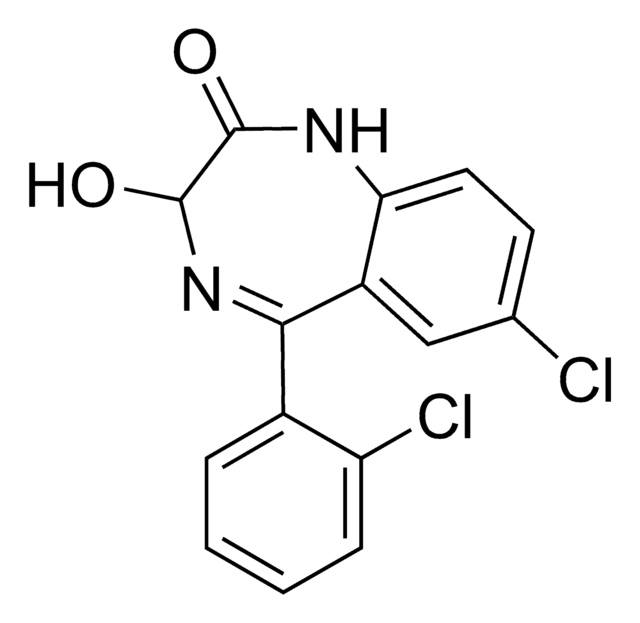
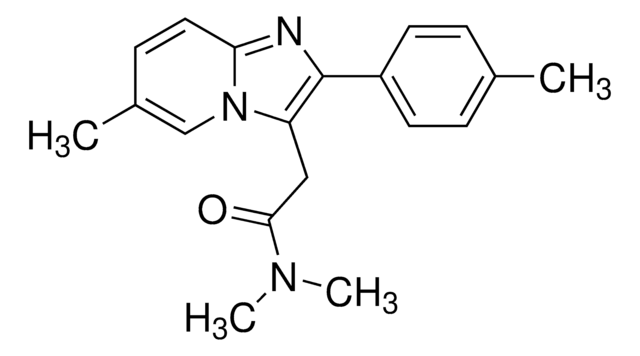
推荐产品
等級
certified reference material
品質等級
形狀
liquid
特點
Snap-N-Spike®/Snap-N-Shoot®
包裝
ampule of 1 mL
製造商/商標名
Cerilliant®
濃度
1.0 mg/mL in methanol
技術
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
應用
clinical testing
形式
single component solution
儲存溫度
−20°C
SMILES 字串
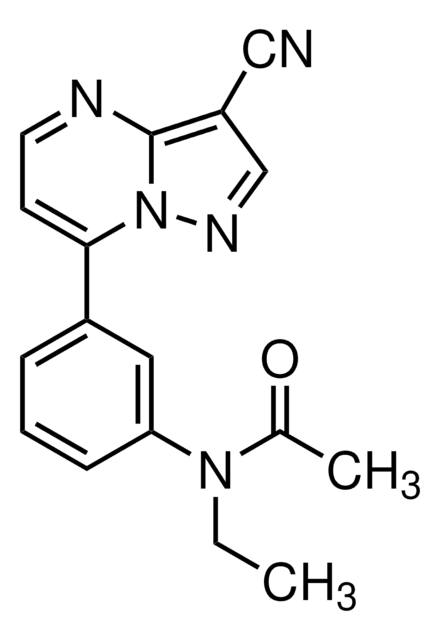
CCN(C(C)=O)c1cccc(c1)-c2ccnc3c(cnn23)C#N
InChI
1S/C17H15N5O/c1-3-21(12(2)23)15-6-4-5-13(9-15)16-7-8-19-17-14(10-18)11-20-22(16)17/h4-9,11H,3H2,1-2H3
InChI 密鑰
HUNXMJYCHXQEGX-UHFFFAOYSA-N
基因資訊
human ... GABRA1(2554) , GABRB1(2560) , GABRG2(2566)
一般說明
應用
- Controlled pore glass beads: Controlled pore glass can be employed for sustainable functionalized metal-organic frameworks for CO2 separation, showcasing the material′s effectiveness in environmental applications due to its high BET surface area which enhances gas adsorption properties (Babar et al., 2021).
- Porous glass for protein adsorption: Mesoporous nano-bioglass for controlled drug release, highlights the application of controlled pore glass with high surface areas in pharmaceutical formulations to enhance the delivery and efficacy of therapeutic agents (Shoaib et al., 2017).
- Surface characterization materials: It can be employed for the contact angle hysteresis of materials with controlled pore structures, to understand the wetting properties of high surface area materials like controlled pore glass, which is critical in various industrial and biomedical applications (Salmas and Androutsopoulos, 2001).
法律資訊
訊號詞
Danger
危險分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
標靶器官
Eyes
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 1
閃點(°F)
49.5 °F - closed cup
閃點(°C)
9.7 °C - closed cup
商品
An rapid method for the siµLtaneous determination of the Z-drugs or sleep aids: zopiclone, zolpidem, and zaleplon is presented here. The need for greater analytical capacity and throughput for the analysis of sleep aid medicines (Z-drugs) in forensic toxicology laboratories can be met by the use of fast Ascentis Express 2.0 micron Fused Core UHPLC Columns.
Separation of Zolpidem phenyl-4-carboxylic acid solution, 500 μg/mL in acetonitrile: water (1:1), ampule of 1 mL, certified reference material; Zopiclone solution, 1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material; Zopiclone-N-oxide solution, 100 μg/mL (Methanol with 1% 1 M HCl), ampule of 1 mL, certified reference material; Zolpidem tartrate solution, 1.0 mg/mL in methanol (as free base), ampule of 1 mL, certified reference material; Zaleplon solution, 1.0 mg/mL in methanol, ampule of 1 mL, certified reference material; N-Desmethylzopiclone
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门