推荐产品
等級
certified reference material
形狀
liquid
特點
Snap-N-Spike®/Snap-N-Shoot®
包裝
ampule of 1 mL
製造商/商標名
Cerilliant®
濃度
100 μg/mL in methanol
技術
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
應用
clinical testing
形式
single component solution
儲存溫度
−20°C
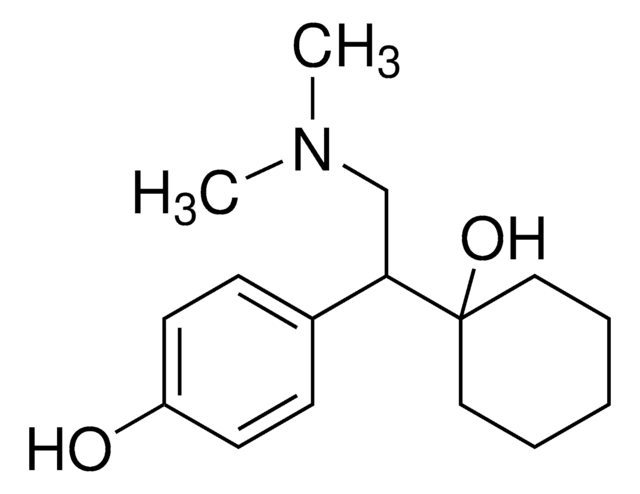
SMILES 字串
OC1=CC=C(C(CN(C)C)C2(O)CCCCC2)C=C1
InChI
1S/C16H25NO2/c1-17(2)12-15(13-6-8-14(18)9-7-13)16(19)10-4-3-5-11-16/h6-9,15,18-19H,3-5,10-12H2,1-2H3
InChI 密鑰
KYYIDSXMWOZKMP-UHFFFAOYSA-N
基因資訊
human ... SLC6A2(6530) , SLC6A4(6532)
正在寻找类似产品? 访问 产品对比指南
一般說明
An analytical reference standard applicable for use as starting material in calibrators or controls for a variety of LC/MS or GC/MS applications such as urine drug testing, forensic analysis, or clinical toxicology. Also known as desvenlafaxine, O-desmethylvenlafaxine is an SNRI antidepressant marketed under the trade name Pristiq® for the treatment of depression. O-desmethylvenlafaxine is also a major urinary metabolite of venlafaxine, an SNRI antidepressant sold as Effexor® or Efexor and used to treat major depressive disorder, generalized anxiety disorder, and other anxiety disorders associated with depression.
法律資訊
CERILLIANT is a registered trademark of Merck KGaA, Darmstadt, Germany
Effexor is a registered trademark of American Home Product Corp.
Pristiq is a registered trademark of Wyeth LLC
Snap-N-Shoot is a registered trademark of Cerilliant Corporation
Snap-N-Spike is a registered trademark of Merck KGaA, Darmstadt, Germany
訊號詞
Danger
危險分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
標靶器官
Eyes
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 1
閃點(°F)
49.5 °F - closed cup
閃點(°C)
9.7 °C - closed cup
Anita H Clayton et al.
The journal of sexual medicine, 10(3), 768-776 (2012-08-22)
The symptoms of major depressive disorder (MDD) include sexual dysfunction, but antidepressant pharmacotherapies are also associated with treatment-emergent sexual dysfunction. These secondary and post hoc analyses evaluated sexual functioning in employed adult outpatients with MDD treated with desvenlafaxine (administered as
JoAnn V Pinkerton et al.
Menopause (New York, N.Y.), 20(1), 38-46 (2012-12-26)
The purpose of this study was to assess the 1-year maintenance of the efficacy of desvenlafaxine 100 mg/day (administered as desvenlafaxine succinate) established on week 12 in a 1-year, double-blind, randomized, placebo-controlled trial in postmenopausal women seeking treatment of bothersome
G Gasser et al.
Chemosphere, 88(1), 98-105 (2012-03-27)
The stereoselectivity of R,S-venlafaxine and its metabolites R,S-O-desmethylvenlafaxine, N-desmethylvenlafaxine, O,N-didesmethylvenlafaxine, N,N-didesmethylvenlafaxine and tridesmethylvenlafaxine was studied in three processes: (i) anaerobic and aerobic laboratory scale tests; (ii) six wastewater treatment plants (WWTPs) operating under different conditions; and (iii) a variety of
R J Cheng et al.
Climacteric : the journal of the International Menopause Society, 16(1), 17-27 (2012-06-01)
To assess effects of desvenlafaxine (administered as desvenlafaxine succinate) on secondary outcomes of mood, climacteric symptoms, and treatment satisfaction in postmenopausal women with moderate to severe menopausal vasomotor symptoms (VMS). A 12-week, multicenter, double-blind, placebo-controlled trial was conducted in postmenopausal
Nakao Iwata et al.
Journal of psychiatric practice, 19(1), 5-14 (2013-01-22)
This study evaluated the efficacy and safety of low-dose desvenlafaxine (administered as desvenlafaxine succinate) in treating major depressive disorder (MDD). Adult outpatients (aged 18 years) in the United States and (aged 20 years) in Japan, who met Diagnostic and Statistical
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持