推荐产品
等級
certified reference material
形狀
liquid
特點
Snap-N-Spike®/Snap-N-Shoot®
包裝
ampule of 1 mL
製造商/商標名
Cerilliant®
drug control
Narcotic Licence Schedule B (Switzerland); psicótropo (Spain); Decreto Lei 15/93: Tabela IV (Portugal)
濃度
1.0 mg/mL in methanol
技術
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
應用
clinical testing
形式
single component solution
儲存溫度
−20°C
SMILES 字串
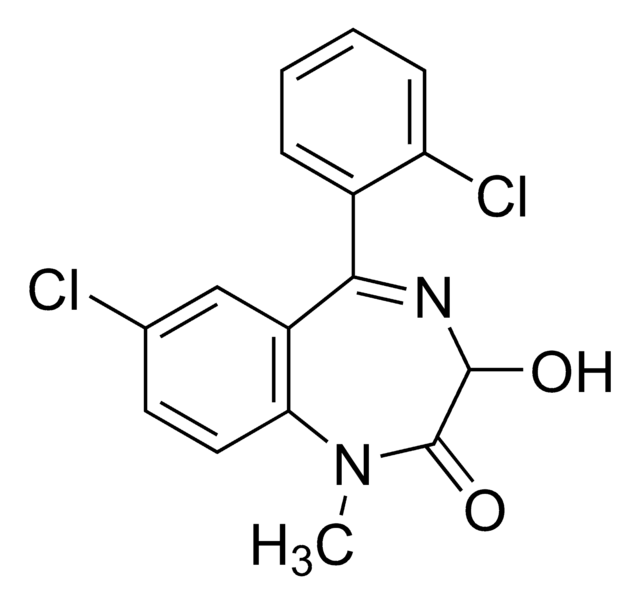
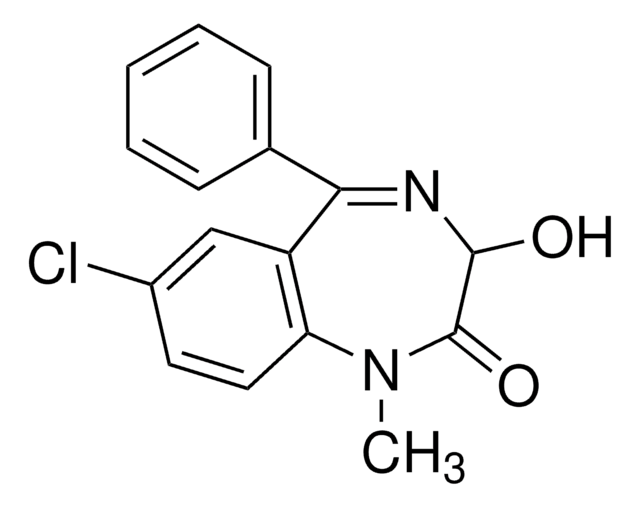
CN1C(=O)C(O)N=C(c2ccccc2Cl)c3cc(Cl)ccc13
InChI
1S/C16H12Cl2N2O2/c1-20-13-7-6-9(17)8-11(13)14(19-15(21)16(20)22)10-4-2-3-5-12(10)18/h2-8,15,21H,1H3
InChI 密鑰
FJIKWRGCXUCUIG-UHFFFAOYSA-N
一般說明
Lormetazepam is a benzodiazepine with sedative-hypnotic, anxiolytic, anticonvulsant, and muscle relaxant effects. This certified solution standard is suitable for use in LC/MS or GC/MS applications for urine drug testing, clinical toxicology, and forensic analysis. Lormetazepam is sold under trade names Loramet and Noctamid for treatment of insomnia.
法律資訊
CERILLIANT is a registered trademark of Merck KGaA, Darmstadt, Germany
Snap-N-Shoot is a registered trademark of Cerilliant Corporation
Snap-N-Spike is a registered trademark of Merck KGaA, Darmstadt, Germany
相關產品
产品编号
说明
价格
訊號詞
Danger
危險分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
標靶器官
Eyes
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 1
閃點(°F)
49.5 °F - closed cup
閃點(°C)
9.7 °C - closed cup
Hervé Allain et al.
European journal of clinical pharmacology, 59(3), 179-188 (2003-05-21)
In elderly patients, both falls and impaired memory are considerable medical problems. Hypnotics, which are frequently administered to this patient group for the treatment of insomnia, should ideally not impair equilibrium or memory functions. This double-blind, randomised, four-way, cross-over study
Francesco Benedetti et al.
International clinical psychopharmacology, 19(5), 311-317 (2004-08-04)
Benzodiazepines can shift the phase of circadian rhythms in mammalian species, but few data are available on their phase-response effects in humans, and on possible links between timing of administration and hypnotic efficacy. Using a placebo-controlled, cross-over design, we evaluated
Inga Katofsky et al.
Sleep medicine, 13(5), 463-468 (2012-02-22)
This study was conducted to evaluate the effectiveness of a cognitive behavioral self-help program (SHP) in combination with pharmacotherapy in patients with primary insomnia in general practice. Patients were recruited from 31 general practitioners (GPs) in the Hamburg area, who
E Nováková
Soudni lekarstvi, 43(1), 2-4 (1998-04-30)
The determination of benzodiazepine derivatives lorazepam and lormetazepam in urine is based on detection of their metabolites 2-amino-2',5-dichlorobenzophenone and 2-methylamino-2'5-dichlorbenzophenone in hydrolyzed urine. Both substances are structurally very similar to the metabolites of diazepam 2-amino-5-chlorbenzophenone and 2-methylamino-5-chlorbenzophenone and their mobility
Patrick Lemmer et al.
Journal of analytical toxicology, 31(4), 224-226 (2007-06-09)
Lormetazepam (Loramet is a benzodiazepine mainly used as an hypnotic to treat insomnia. Lorazepam (Temesta) is used as an anxiolytic, tranquilizer, sedative, and anticonvulsant, and it is the major metabolite of lormetazepam. In this study, we designed a method to
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门