推荐产品
等級
certified reference material
品質等級
形狀
liquid
特點
Snap-N-Spike®/Snap-N-Shoot®
包裝
ampule of 1 mL
製造商/商標名
Cerilliant®
濃度
1.0 mg/mL in methanol
技術
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
應用
clinical testing
形式
single component solution
儲存溫度
2-8°C
SMILES 字串
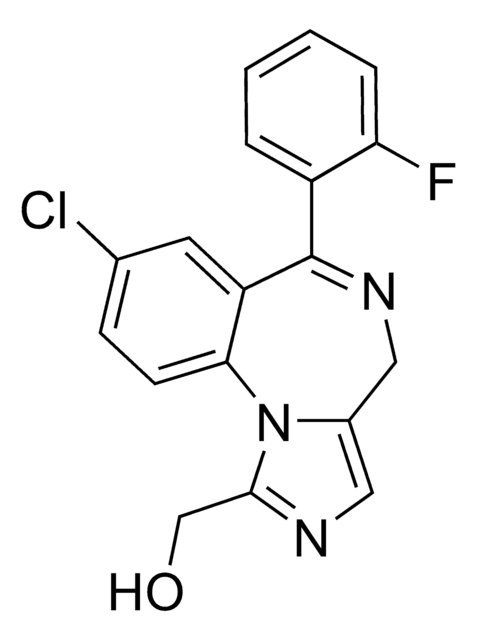
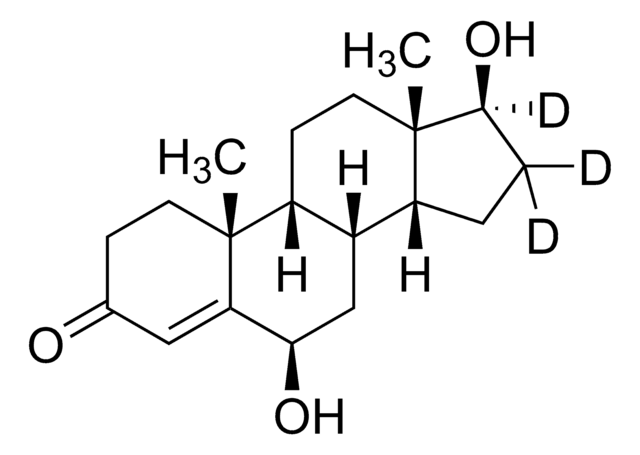
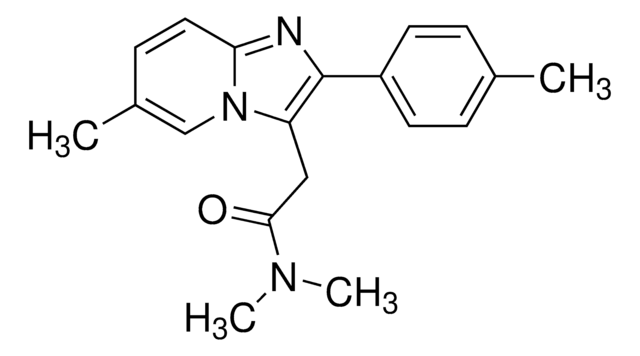
OCc1ncc2CN=C(c3ccccc3F)c4cc(Cl)ccc4-n12
InChI
1S/C18H13ClFN3O/c19-11-5-6-16-14(7-11)18(13-3-1-2-4-15(13)20)22-9-12-8-21-17(10-24)23(12)16/h1-8,24H,9-10H2
InChI 密鑰
QHSMEGADRFZVNE-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
α-羟基咪达唑仑是苯二氮卓咪达唑仑在尿液和血液中的活性主要代谢产物。咪达唑仑以Dormicum、Hypnovel®和Versed商品名销售,并已作为治疗失眠和惊厥的镇静剂和治疗方法。该认证的加标溶液®适合用作从法医分析和临床毒理学研究到尿液药物检测的各种LC/MS或GC/MS应用的校准品或对照品的起始物料。
法律資訊
CERILLIANT is a registered trademark of Merck KGaA, Darmstadt, Germany
CERTIFIED SPIKING SOLUTION is a registered trademark of Cerilliant Corporation
Hypnovel is a registered trademark of Hoffman-LaRoche & Co. AG
Snap-N-Shoot is a registered trademark of Cerilliant Corporation
Snap-N-Spike is a registered trademark of Merck KGaA, Darmstadt, Germany
相關產品
产品编号
说明
价格
訊號詞
Danger
危險分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
標靶器官
Eyes
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 2
閃點(°F)
49.5 °F - closed cup
閃點(°C)
9.7 °C - closed cup
其他客户在看
Kristin Samuelsson et al.
Xenobiotica; the fate of foreign compounds in biological systems, 42(11), 1128-1137 (2012-05-31)
The pharmacokinetics and biotransformation of midazolam were investigated following single oral doses of 0.1, 1 and 10 mg/kg to chimeric mice with humanised livers (PXB mice) and to severe combined immunodeficient (SCID) mice used as controls. Pharmacokinetic analysis, on whole
Varun Garg et al.
Journal of clinical pharmacology, 52(10), 1566-1573 (2011-12-14)
In this open-label study, 24 healthy volunteers received a single intravenous (IV) dose of 0.5 mg of midazolam on day 1 and a single oral dose each of 2 mg of midazolam and 0.5 mg of digoxin on day 3.
Theo de Boer et al.
Biomedical chromatography : BMC, 25(10), 1112-1123 (2011-02-03)
An early clinical development study (phase I) was conducted to determine the usefulness of dried blood spot (DBS) sampling as an alternative to venous sampling for phenotyping and genotyping of CYP450 enzymes in healthy volunteers. Midazolam (MDZ) was used as
Stephanie Katzenmaier et al.
European journal of clinical pharmacology, 66(11), 1137-1141 (2010-08-04)
Midazolam metabolic clearance to 1'-hydroxymidazolam is an accurate measure of CYP3A activity which requires extensive plasma and urine sampling. The objective of this study was to find a new limited sampling strategy (LSS) to predict midazolam metabolic clearance to 1'-hydroxymidazolam
David Hostler et al.
Drug metabolism and disposition: the biological fate of chemicals, 38(5), 781-788 (2010-02-19)
The clinical use of therapeutic hypothermia has been rapidly expanding due to evidence of neuroprotection. However, the effect of hypothermia on specific pathways of drug elimination in humans is relatively unknown. To gain insight into the potential effects of hypothermia
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门